Ants of the Osa Biodiversity Center
Report by John T. (Jack) Longino, The Evergreen State College.
The Evergreen State College Tropical Rainforest Program, 2008, had the pleasure of staying at the Osa Biodiversity Center, 8-13 March 2008. We carried out a major ant sampling program, using miniWinkler sampling. Also I carried out a small amount of additional collecting. This report summarizes the results of the Winkler project and provides a species list of ants encountered at the station.
The Winkler project was designed to measure the effect of a modification of the method. Normally a miniWinkler samples is taken by chopping a meter square area of forest floor with a machete and then immediately sifting the chopped material. This sifted material is later hung in a Winkler bag for extraction. We asked whether chopping and then waiting a while before sifting would alter the sampling efficiency, as measured by number of species per plot. We also wanted to know whether chopping would attract army ants, with the prediction that plots with a delay between chopping and sifting would have a higher incidence of army ants.
Participants

The 2008 Tropical Rainforests class: Amber Carver, D. J. Cox, Christine Davis, Oren Grimm, Katherine Halstead, Edward Holbrook, Jameson Honeycutt, Molly Hukari, Kira Kranzler, Ryan Kruse, Benjamin Lee, Aimee Machiels, Neal Marks, Corrie McAliley, Sarah Michel, Amber Mount, Michelle Olsen, David Peterson, Samantha Price, Nick Smith, Jessica Starrett, Kendra Steadman, Lauren Troyer, Adam Wicks-Arshack (Faculty and staff: Paul Butler, Melquisedec Gamba-Rios, Jack Longino).

The Project LLAMA sample-sorting group, left to right: Brittany Broyles, D. J. Cox, Jesse McAlpine, Duke Brady (far right Jack Longino).
Methods
On 10 March we took 60 miniWinkler samples. Six groups of 3-4 students were stationed at 50m intervals along the upper part of Trail #13, which is an area of primary forest. Each group laid out a transect due west (the trail runs roughly north-south) and took 10 miniWinkler samples at 5m intervals. Each group had five samples that were chopped at about 8am and then sifted at about 10am (2 hours later), and five samples that were chopped and sifted during the second time interval. Thus the actual sifting took place at about the same time for all samples, the only difference between the two groups being that one was chopped 2 hours before sifting and one chopped immediately prior to sifting. The order of sample treatments was randomized within each sifting group.
The samples were carried down to the station and hung in miniWinkler bags that afternoon. They were left to extract for three days, after which samples were bagged and returned to the Longino Lab at Evergreen. The crew of the LLAMA project (Brittany, D. J., Duke, Jesse) sorted all the ants from the samples. I then identified the ants in each sample. The results are based only on workers (species for which only queens were found were ignored). Also, a scattering of Crematogaster erecta, a dominant arboreal ant on the scaffolding where the samples were hung, occurred in the samples. These were all assumed to be contaminants and were discarded.

Sixty MiniWinkler samples at Osa Biodiversity Center, hanging in miniWinkler bags.
Results
The samples were remarkably diverse. There was a total of 115 species overall.
There were four samples with an army ant, Labidus coecus. Two were from delay samples, two from no-delay samples. So the delay treatment did not result in a higher incidence of army ants.
The average number of species per sample was 16. There was no significant difference in number of species between the two treatments. Thus chopping and delaying has no effect on the efficiency of species capture.
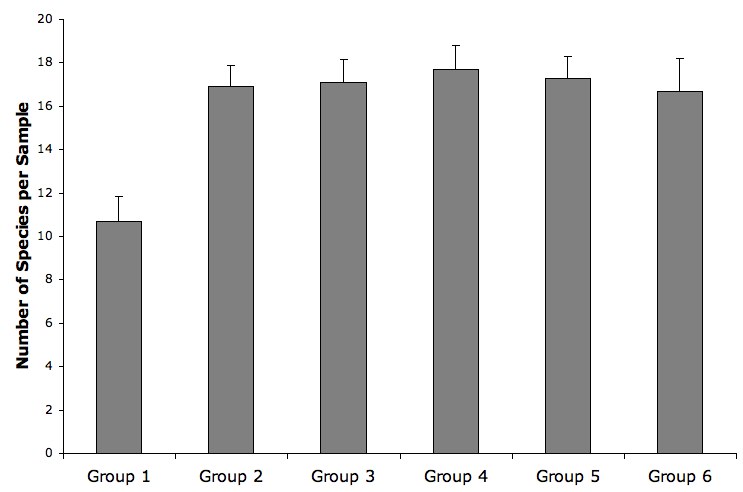
The figure above shows the means and standard errors of species per sample for the six sifting groups. Group 1 had a within-sample richness distinctly lower than the other groups (1-way ANOVA, p < 0.001). This could have been due to some kind of habitat discontinuity or a problem with technique in group 1.
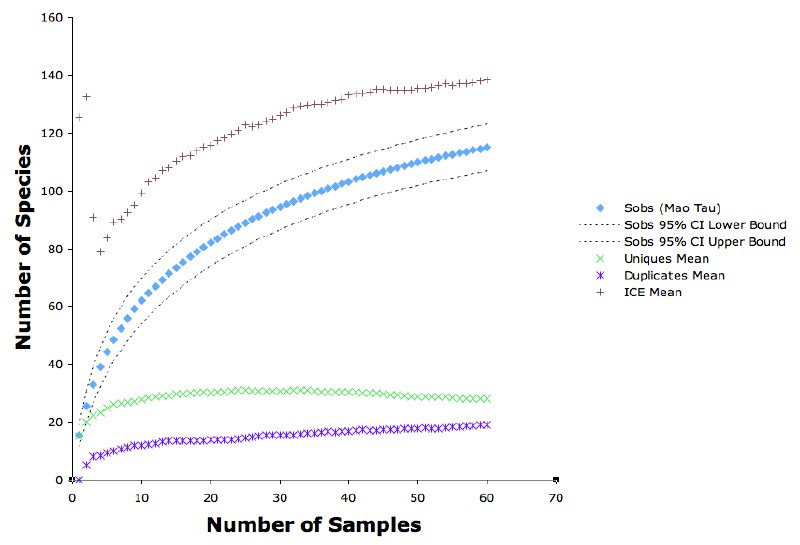
The figure above shows the species accumulation curve and some other associated measures (calculated using EstimateS). The curve is roughly logarithmic, not asymptotic. The richness estimator ICE is perhaps approaching an asymptote at about 140 species.
Ant List
The following is a list of all ants collected during the March visit. Species with a number in parentheses after the name were collected in the quantitative Winkler sampling; the number is the percent of the 60 samples in which the species occurred. Species are in alphabetical order by genus, and in descending order of abundance within genera. Species are linked to web pages on the Ants of Costa Rica website. Note that there are almost no arboreal ants; no collecting of arboreal ants was done while at the site, but there is certainly a rich fauna of Azteca, Crematogaster, Pseudomyrmex, Camponotus, and other arboreal groups.
Discoveries of particular interest include: (1) one of the most common litter Pheidole species (JTL-157) is an undescribed species; (2) one specimen is an undescribed species of Rogeria; (3) two species in the Pachycondyla ferruginea complex (P. ferruginea panamensis and P. JTL-016) were found; this evidence of sympatry is important for inferring species boundaries in the group; (4) Ectatomma edentatum is a largely South American species at the extreme northern limit of its range in Costa Rica; it has been collected very few times in Costa Rica; (5) a very distinctive new morphospecies of Hypoponera was found, one of the largest Hypoponera species I have seen.
Which species are missing? Species common elsewhere and notable for being absent on the Osa are Paraponera clavata and Ectatomma ruidum. It is also interesting that no Thaumatomyrmex or Tatuidris are known from the southern Pacific lowlands of Costa Rica.
Acanthognathus ocellatus (3)
Acropyga exsanguis (5)
Adelomyrmex myops (22)
Adelomyrmex silvestrii (18)
Adelomyrmex longinoi (7)
Aphaenogaster phalangium (na)
Apterostigma pilosum (8)
Apterostigma dentigerum (3)
Carebara urichi (8)
Carebara reina (3)
Carebara brevipilosa (2)
Cerapachys JTL-006 (na)
Cerapachys neotropicus (5)
Crematogaster erecta (27)
Cerapachys JTL-005 (2)
Crematogaster tenuicula (27)
Crematogaster nigropilosa (13)
Crematogaster snellingi (10)
Crematogaster carinata (5)
Cyphomyrmex rimosus s.l. (23)
Cyphomyrmex costatus (15)
Cyphomyrmex salvini (12)
Discothyrea humilis (na)
Discothyrea denticulata (3)
Discothyrea JTL-005 (3)
Eciton crassinode (na)
Ectatomma edentatum (3)
Ectatomma tuberculatum (2)
Eurhopalothrix gravis (2)
Gnamptogenys JTL-002 (cf. interrupta) (5)
Gnamptogenys tornata (5)
Gnamptogenys minuta (3)
Gnamptogenys annulata (2)
Gnamptogenys haenschi (2)
Hylomyrma dentiloba (8)
Hypoponera JTL-007 (55)
Hypoponera parva (32)
Hypoponera JTL-013 (7)
Hypoponera JTL-021 (2)
Hypoponera opacior (2)
Labidus coecus (7)
Lachnomyrmex plaumanni (35)
Leptogenys pusilla (3)
Linepithema angulatum (3)
Megalomyrmex drifti (10)
Megalomyrmex silvestrii (5)
Mycocepurus smithii (2)
Myrmicocrypta sp. (3)
Octostruma balzani s.l. (72)
Octostruma iheringi (3)
Odontomachus bauri (2)
Odontomachus meinerti (2)
Pachycondyla JTL-003 (cf. harpax) (13)
Pachycondyla harpax (12)
Pachycondyla constricta (8)
Pachycondyla ferruginea panamensis (7)
Pachycondyla JTL-011 (3)
Pachycondyla impressa (2)
Pachycondyla JTL-016 (2)
Pachycondyla stigma (2)
Pachycondyla verenae (2)
Paratrechina steinheili (52)
Paratrechina guatemalensis (15)
Pheidole multispina (70)
Pheidole JTL-157 (62)
Pheidole rugiceps (60)
Pheidole ruida (33)
Pheidole pugnax (25)
Pheidole specularis (25)
Pheidole prolixa (23)
Pheidole otisi (18)
Pheidole simonsi (17)
Pheidole anastasii (15)
Pheidole glomericeps (10)
Pheidole flavens (7)
Pheidole celaena (5)
Pheidole indistincta (5)
Pheidole veletis (3)
Pheidole arachnion (2)
Pheidole carapuna (2)
Pheidole potosiana (2)
Pheidole susannae (2)
Prionopelta modesta (5)
Pseudomyrmex boopis (2) [no species page]
Pyramica fridericimuelleri (na)
Pyramica gundlachi (67)
Pyramica brevicornis (48)
Pyramica zeteki (35)
Pyramica aethegenys (8)
Pyramica subedentata (3)
Pyramica alberti (2)
Rogeria foreli (na)
Rogeria leptonana (20)
Rogeria neilyensis (17)
Rogeria creightoni (13)
Rogeria inermis (8)
Rogeria tonduzi (7)
Rogeria belti (3)
Rogeria gibba (2)
Rogeria JTL-005 (2)
Sericomyrmex amabilis (10)
Solenopsis altinodis (na)
Solenopsis JTL-005 (75)
Solenopsis JTL-021 (42)
Solenopsis terricola (38)
Solenopsis JTL-007 (27)
Solenopsis geminata (10)
Solenopsis JTL-009 (7)
Solenopsis subterranea (7)
Solenopsis JTL-008 (3)
Solenopsis JTL-025 (3)
Solenopsis JTL-012 (2)
Stenamma JTL-006 (32)
Strumigenys elongata (18)
Strumigenys longispinosa (8)
Strumigenys cordovensis (5)
Strumigenys micretes (2)
Trachymyrmex cornetzi (na)
Trachymyrmex bugnioni (3)
Tranopelta gilva (3)
Typhlomyrmex pusillus (2)
Wasmannia auropunctata (28)
Page author:
John T. Longino, The Evergreen State College, Olympia WA 98505
USA.longinoj@evergreen.edu
Date of this version: 10 April 2008.
Go to Longino Homepage




