
Figure 1. Anterior face of worker posterior femur, showing contrasting pilosity of Aphaenogaster phalangium and A. araneoides. Aphaenogaster phalangium has erect setae on all faces. Aphaenogaster araneoides has erect setae on ventral surface only.
| Genus List | Species List | Key to Species |
[Most of this page is from Longino and Cover 2004. Click here for specimen data for this paper.]
Forested habitats of Central America are home to ants of the Aphaenogaster phalangium complex. Within the context of the New World Aphaenogaster fauna, the workers of the phalangium complex are uniquely identified by a combination of head and propodeum characters. The portion of the head posterior to the eyes is drawn out and tapers to a strongly constricted neck, beyond which the head flares out into a pronounced collar. The propodeum lacks spines, although it may be weakly tuberculate.The workers are large, with long spindly legs. Intrapopulational variation in conspicuous morphological features is always very low, but dramatic character shifts occur over short distances (Longino and Cover 2004). Members of the complex are a common element of many lowland rainforest sites, where solitary foragers are frequent on the forest floor. They are timid ants that run from any threat, and they are decidedly comical with their large heads on implausibly narrow necks. The nests are in small chambers in the ground, and colonies maintain multiple empty nests, moving among them (McGlynn et al. 2002, 2003).
A character with two discrete states, and the one on which we base our definitions of species, involves pilosity on the femora of workers (Fig. 1). Aphaenogaster phalangium is a "pilose" form, and A. araneoides is a "non-pilose" form. Aphaenogaster phalangium has femora with short stiff erect setae that cover all surfaces. In contrast, A. araneoides has the dorsal and lateral faces of the femora with only very fine, fully appressed setae, and erect setae are confined to the ventral surface. In most cases there is also a pilosity difference on the mesepisternum. Aphaenogaster phalangium usually has ten or more erect setae scattered along the full length of the mesepisternum. In contrast, A. araneoides is usually devoid of erect setae on the mesepisternum, or with at most one or two at the ventral border. Rarely (see A. brevicollis, a junior synonym of A. araneoides) there is a row of about five setae along the posterior border. The two species exhibit a largely parapatric distribution with a narrow zone of sympatry (Fig. 2). Aphaenogaster phalangium occurs in a narrow strip along the mid-elevation Pacific slope of Costa Rica's cordilleras, fanning out into the wet Pacific lowlands of southwestern Costa Rica, and extending into Panama as far as Barro Colorado Island and the Darien. Aphaenogaster araneoides is the Atlantic slope counterpart, also occurring in the mid-elevation cordilleras of Costa Rica, but fanning out into the Atlantic lowlands, with isolated records from El Salvador and Honduras.

Figure 1. Anterior face of worker posterior femur, showing contrasting pilosity of Aphaenogaster phalangium and A. araneoides. Aphaenogaster phalangium has erect setae on all faces. Aphaenogaster araneoides has erect setae on ventral surface only.
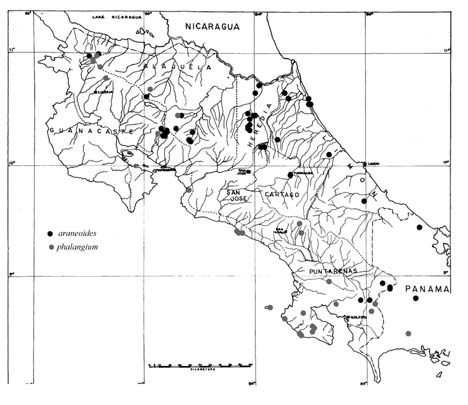
Figure 2. Distribution map of Aphaenogaster phalangium and A. araneoides. Specimens examined are the combined holdings of the Museum of Comparative Zoology at Harvard, the Instituto Nacional de Biodiversidad (INBio) in Costa Rica, and the private collection of the senior author. For specimen data click here. Not included on the map are specimens from Honduras, El Salvador, and central Panama.
In the zone of sympatry collections are sparse, but one locality shows evidence of discrete sympatric species. In the Monteverde area of the Cordillera de Tilarçn, one colony of A. phalangium and eight separate worker samples of A. araneoides have been collected. Elsewhere, collections of both species have been taken from closely approximated localities (Fig. 2). For example, in the Cordillera de Guanacaste, an A. phalangium worker was collected from Cerro Cacao, at 1500m, while an A. araneoides worker was collected from Maritza Biological Station, at 600m elevation and only 6 km from Cerro Cacao.
A character that we interpret as being intraspecifically variable is the sculpture on the fourth abdominal tergite. This tergite may be completely smooth and shining or variably matte and dull. For A. phalangium, specimens from the montane sites in the cordilleras of Guanacaste, Tilarçn, and Talamanca have the tergite smooth and shining or matte anteriorly grading to smooth posteriorly. Specimens from the lowlands, including the southern Pacific lowlands of Costa Rica and the population on Barro Colorado Island, have the tergite completely matte. For A. araneoides, the pattern is more or less the same. Specimens from the Costa Rican cordilleras and the one collection from Honduras tend to have the tergite shiny; specimens from the Atlantic lowlands and the collection from El Salvador have the tergite matte.
The shape of the neck, although difficult to quantify, shows subtle variation (Fig. 3). In lateral view, the neck may be (1) very strongly constricted and with a strongly developed dorsal flange, or (2) relatively weakly constricted with a reduced dorsal flange. In the first instance the profile of the head looks like a bottle or flask with rounded shoulders and a separate neck; in the second it looks like a flask that tapers to the opening. Aphaenogaster phalangium always has the strongly constricted neck. Specimens of A. araneoides from the Atlantic lowlands of Costa Rica up to the Peľas Blancas Valley show the weakly constricted neck, while those from Monteverde and elsewhere in the central cordilleras have the strongly constricted neck of A. phalangium. Strong character variation occurs over small spatial scales. In the Peľas Blancas Valley workers have the same neck shape as workers from La Selva and the slopes of the Cordillera Volcanica Central but the shiny abdominal tergites similar to material of A. araneoides from Monteverde. In Monteverde, only 10km from Peľas Blancas, not only are the abdominal tergites shiny, the neck shape is very different too, matching both A. araneoides and A. phalangium material from further north.

Figure 3. Lateral view of worker head, Aphaenogaster phalangium complex.
The propodeum may have short posterodorsal tubercles (where the propodeal spines would be in other Aphaenogaster), or these may be completely lacking. Propodeal tubercles occur on specimens from the Atlantic slope of the Cordillera Volcanica Central south to the Rio Sixaola on the border between Costa Rica and Panama. Specimens from north and south (the Peľas Blancas Valley and Changuinola, Panama, respectively) lack the tubercles.
The low number of males in collections reduces knowledge of male character variation, but some observations are warranted. We have examined males of A. phalangium from Corcovado, Quepos (a site on the Pacific coast just north of Corcovado), and Barro Colorado Island, and males of A. araneoides from La Selva, two other Atlantic lowland sites south of La Selva, and Monteverde. The leg pilosity character does not differentiate the species, because femora of both species have appressed setae on the dorsal and lateral faces (although the femoral pilosity of A. phalangium males is not as strongly appressed). The males often retain the pilosity differences on the mesepisternum. The A. phalangium males have sparse setae scattered over the katepisternum and often on the anepisternum as well. These setae vary from being thickened and easily seen to very fine, delicate, and difficult to see. In contrast, the mesepisternum of A. araneoides males is usually entirely or almost entirely devoid of setae, with at most one or two at the ventral border. However, the males of Forel's A. brevicollis (see below) have setae on the katepisternum, yet the worker leg pilosity places them in A. araneoides. The fourth abdominal tergite of all males we have examined has been entirely smooth and shiny, or with at most a small amount of shagreening near the postpetiolar insertion. The males vary intraspecifically in the size of the compound eyes (Fig. 4), and like some worker characters eye size does not seem to correlate with species boundaries. Male eyes are small in A. araneoides from Monteverde, A. phalangium from Corcovado, and A. araneoides from Changuinola. Male eyes are large and bulging in A. phalangium from Barro Colorado Island and A. araneoides from Bataan and La Selva, both Costa Rican Atlantic lowland sites. The shift from small to large eyes across the Rio Sixaola appears to correlate with the shift from the non-tuberculate to tuberculate condition in workers.
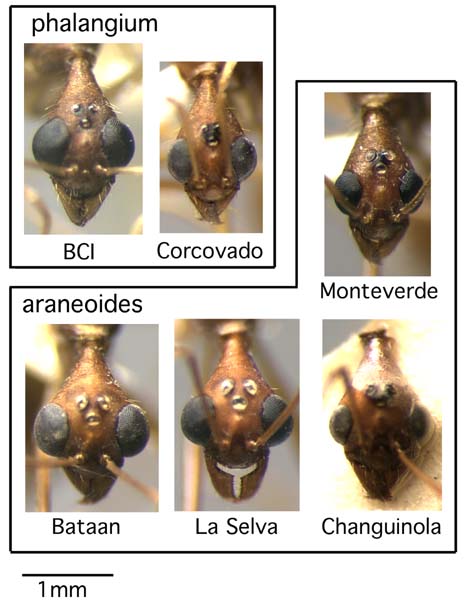
Figure 4. Face view of male, Aphaenogaster phalangium complex.
Queens
McGlynn et al. (2002) found that colonies of A. araneoides at La Selva Biological Station never contained alate queens, but instead contained one or two ergatoid queens. In our examination of museum material we have never seen an alate queen, and four nest series (two of A. araneoides collected by E. O. Wilson at La Selva, one of A. phalangium collected by G. C. Wheeler on Barro Colorado Island, and one of A. phalangium collected by Longino from Monteverde) contain single ergatoid queens. In each of these collections one adult female has an allometrically enlarged postpetiole and fourth gastral tergite (Fig. 5). Also the metanotal groove is more deeply impressed relative to workers. There are no traces of wing scars or the sclerites associated with wings. The La Selva individuals have an ocellar triangle with distinct ocelli. The Barro Colorado individual lacks ocelli but there is a small depression where the median ocellus would be. The Monteverde specimen has no trace of ocellus or depression.

Figure 5. Dorsal view of postpetiole and gaster, Aphaenogaster phalangium complex, contrasting ergatoid queen and worker.
Discussion
If there are two species as revealed by the sympatric leg pilosity character, then the abdominal tergite sculpture, head shape, and propodeal tubercle characters show discordant clinal variation that crosses species boundaries. Population sampling for molecular markers should be the next step in testing these species definitions. Patterns of parallel or convergent geographic variation among different species are known for other taxa, including the well-known case of parallel mimicry races in Heliconius butterflies (Turner 1981). Ward (1999) discovered a similar phenomenon when clarifying species boundaries among Neivamyrmex army ants (Ecitoninae) in the southwestern United States. Neivamyrmex nigrescens occurs broadly across the southwestern United States and N. californicus occurs in California and Baja California. The two are sympatric in northern California. Neivamyrmex nigrescens shows pronounced geographic variation, with the northwestern populations strongly differentiated from the southeastern populations and convergent on N. californicus. Ward discovered that many collections of nigrescens had been misidentified as californicus because of the morphological convergence in the zone of sympatry.
This parallels what we have discovered in the zone of sympatry of A. phalangium and A. araneoides. The causes of these cases of interspecifically correlated geographic variation are unexplored, but two hypotheses are introgression between the two species and convergence due to natural selection. In the first case there may be frequent gene flow among the two species, such that populations that are in contact share genes that determine such traits as abdominal sculpture and neck shape. In this case the meaning of the species boundaries can be questioned. Admittedly the decision to use leg pilosity as the main separatory character was based largely on its discreteness. Specimens could be unambiguously sorted by the character. It could be that leg pilosity has a simple genetic basis and is itself an intraspecific polymorphism showing clinal variation, and independent of clines for other characters such as abdominal sculpture and neck shape. Alternatively the two species may be well separated genetically, but selection has generated parallel changes in both species. Perhaps selection favors smooth abdomens in montane areas and matte abdomens in lowland habitats.
We have demonstrated strongly developed intraspecific geographic variation similar to that seen in Ecitoninae. It is well-known that army ants have wingless queens and that all long-distance gene flow has to occur through males. Queens of the Aphaenogaster phalangium complex are also wingless. The restricted gene flow in lineages with low-vagility queens may contribute to the excessive geographic variation seen in these and other ant lineages.
The nature of biological diversity and the subjectivity of the taxonomic process illustrate the difficulties in obtaining global tallies of species richness. We have taken six names and made them into two. Another taxonomist might recognize each geographic variant of both A. phalangium and A. araneoides as a distinct species. We can imagine a "splitter" generating at least ten taxa with the material we examined. Using this study as a guide, different approaches to mapping biodiversity (O'Hara 1993) could result in global counts of species richness that differ by a factor of five.
Literature Cited
Longino, J. T., and S. Cover. 2004. A Revision of the Aphaenogaster phalangium complex (Hymenoptera: Formicidae: Myrmicinae). Zootaxa 655:1-12.
McGlynn, T. P., Hoover, J. R., Jasper, G. S., Kelly, M. S., Polis, A. M., Spangler, C. M., and Watson, B. J. (2002) Resource heterogeneity affects demography of the Costa Rican ant Aphaenogaster araneoides. Journal of Tropical Ecology 18, 231-244.
McGlynn, T. P., Shotell, M. D., and Kelly, M. S. (2003) Responding to a variable environment: Home range, foraging behavior, and nest relocation in the Costa Rican rainforest ant Aphaenogaster araneoides. Journal of Insect Behavior 16, 687-701.
O'Hara, R. J. (1993) Systematic generalization, historical fate, and the species problem. Systematic Biology 42, 231-246.
Turner, J. R. G. (1981) Adaptation and evolution in Heliconius: a defense of NeoDarwinism. Annual Review of Ecology and Systematics 12, 99-121.
Ward, P. S. (1999) Deceptive similarity in army ants of the genus Neivamyrmex (Hymenoptera: Formicidae): taxonomy, distribution and biology of N. californicus (Mayr) and N. nigrescens (Cresson). Journal of Hymenoptera Research 8, 74-97.
Page authors:
John T. Longino, The Evergreen State College, Olympia WA 98505 USA. longinoj@evergreen.edu
Stefan Cover, Museum of Comparative Zoology, Harvard University, Cambridge MA 02138 USA. scover@oeb.harvard.edu