Ants of the Osa Biodiversity Center, Updated
Report by John T. (Jack) Longino, The Evergreen State College.
Two years ago the Evergreen State College Tropical Rainforest Program visited the Osa Biodiversity Center and carried out a program of litter ant sampling using miniWinkler samples (see report). The 2010 program returned to OBC and repeated the sampling in the same spot.
This year's group worked at OBC from 6-11 March 2010. There was an experimental element to the project: we tested whether baiting plots prior to sifting would increase sampling efficiency (in terms of species richness) and whether it would increase the capture rate of Pheidole major workers.
Participants

The 2010 Tropical Rainforests class: Avery Adams, Brendon Boudinot, Casey Broderick, Jill Cooper, Oliver Dibble, Somerset Fetter, Kym Foley, Annie Forman, Brian Gallagher, David Isomaki, Mary Lightle, Gerin Love, Heidi Montez, Britt O'Leary, Jasmine Reppen, Sara Rosewall, Andrew Scott-Busenbark, Jared Smith, Alexandra Stefancich, Dana Swarth, Jacqueline Taylor, Courtney Thomas, Carlos Duran Westergaard (Faculty and staff: Alison Styring, Jack Longino, Mark Wainwright).
Methods
On 7 March we took 60 miniWinkler samples. We went to the same section of trail where samples were taken in 2008, and used the same methods to place the 60 samples. Six groups of 3-4 students were stationed at 50m intervals along the trail, which is an area of primary forest. Each group laid out a transect due west (the trail runs roughly north-south) and took 10 miniWinkler samples at 5m intervals. For each transect group, each of the 10 sample plots was randomly assigned to one of two treatments, baiting and control, such that there were 5 baiting samples and 5 controls. For baiting sample plots, two cookies (Pecan Sandies) were crumbled over the 1 square meter plot. Control plots were left unmanipulated. After 1 hour, all plots were chopped with machete and sifted, following the usual miniWinkler procedure. Sampling was completed by noon and samples were transported to the station. The 60 miniWinkler bags were hung in the laboratory building. They were hung haphazardly on six suspended rails and not grouped by transect. Samples were left to extract for 3 days.
There were pest ants in the building, mainly Tetramorium bicarinatum and Solenopsis geminata. The presence of these in the samples could be contaminants.
The samples were returned to the Longino Lab at Evergreen. All ants were removed from the samples and sorted (Thanks to Brendon Boudinot, Casey Broderick, Jill Cooper, Sarah Rosewall, and Ali Stefancich from the Tropical Rainforests class, and Abby Aspee, Cheryl Braun, and Sara Roberts from the Invertebrate Zoology and Entomology class for helping with sample sorting). Unlike the 2008 samples, some difficult genera were not sorted to species. Nylanderia was treated as a single taxon. Hypoponera was treated as two: H. parva and all other Hypoponera. Solenopsis was treated as two taxa: S. geminata and all other Solenopsis. Thus "species" in the report are a combination of species and groups of species. The results are based only on workers (species for which only queens were found were ignored). One of the sample vials was lost during processing, resulting in a sample size of 59.
Results
The total number of ants in the sample was approximately normal with the exception of one outlier (Fig. 1). The outlier was removed from further analysis.
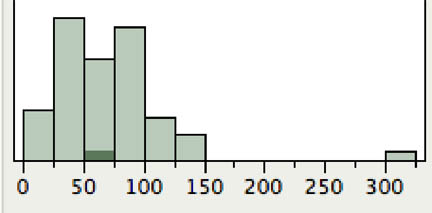
Figure 1. Distribution of number of ants per sample. Horizontal axis is number of ants, vertical axis is number of samples. The outlier was a sample with the army ant, Labidus coecus.
There was no significant difference between the treatments in number of ants per plot, although there was a weak trend in the expected direction of more ants in plots with the cookie treatment (Fig. 2). There were also no significant differences for Pheidole major workers, non-pselaphine Staphylinidae, Curculionidae, Araneae, and Chilopoda.
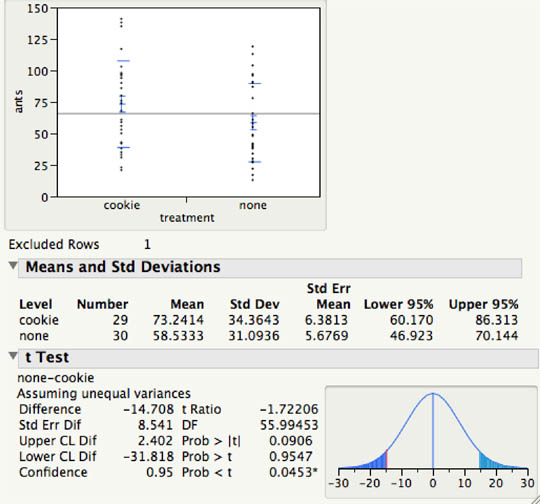
Figure 2. Output from two-sample t-test, testing whether plots with cookie bait had fewer or more ants (i.e., two-tailed test) than plots without augmentation. The two-tailed p-value was 0.09.
There was no significant difference among the six transect groups (a good sign!).
The samples in 2010 contained 91 species, of which 31 were uniques (found in only one sample). The 2008 samples contained 101 species and 23 uniques. The combined samples from both years contained 126 species and 32 uniques.
The species accumulation curves, scaled to number of species occurrences, are identical for the two years (Fig. 3). The combined curve is slightly higher, suggesting a small change in community composition between 2008 and 2010.
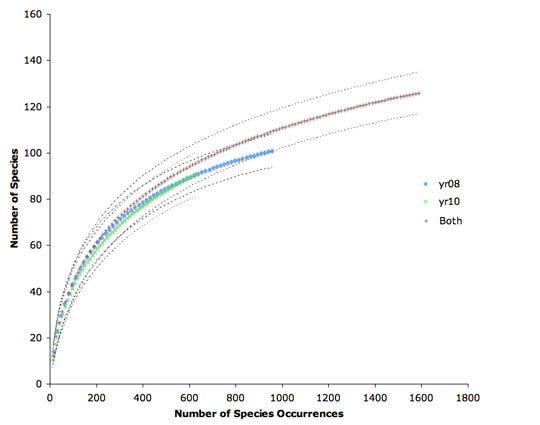
Figure 3. Species accumulation curves for ants in MiniWinkler samples, for the two separate years (60 samples in 2008 and 59 samples in 2010) and for the combined data. Dotted lines are 95% confidence intervals.
The total number of species occurrences was lower this year, 855 in 2008 versus 620 in 2010. Several of the litter species that were dominant two years ago had substantially lower frequency this year.
Ant List
Updating the list from two years ago, Pheidole JTL-157 has been described and has the name Pheidole carinote. Pyramica JTL-019 is a highly distinctive new species. Several specimens of Thaumatomyrmex atrox were an exciting find. This is the first report of the genus for OBC, and it is a species that is widespread in South America but extremely rare in Central America. In Costa Rica, it was previously known from a single specimen from La Selva Biological Station. Another very rare find was multiple series of Probolomyrmex boliviensis, a cryptic soil ant.
Additional species added to the OBC list are:
Azteca instabilis
Basiceros manni
Camponotus sericeiventris
Cerapachys JTL-002
Crematogaster sotobosque
Leptogenys JTL-019
Megalomyrmex symmetochus
Neivamyrmex adnepos
Nesomyrmex echinatinodis
Nesomyrmex pleuriticus
Nomamyrmex hartigii
Pachycondyla unidentata
Pheidole colobopsis
Pheidole gauthieri
Platythyrea punctata
Probolomyrmex boliviensis
Pyramica JTL-019
Thaumatomyrmex atrox
Wasmannia scrobifera
Page author:
John T. Longino, The Evergreen State College, Olympia WA 98505
USA.longinoj@evergreen.edu
Date of this version: 17 September 2010.
Go to Longino Homepage



