| Genus List | Key to Species | Species list |
Wasmannia of Costa Rica
The genus Wasmannia is endemic to the Neotropics. Its most famous member is W. auropunctata, the little fire ant. This species does very well in synanthropic habitats throughout the Neotropics and has been introduced to tropical locales throughout the world, where it often becomes a severe pest ant. Other species in the genus are few, and all of them are uncommon relative to their famous relative.
The genus Wasmannia was established in 1893 by Forel. The most recent ant classification (Bolton 2003) places Wasmannia in the tribe Blepharidattini, within the attine tribe group. Bolton proposes as an autapomorphy for the attine tribe group the "anterior clypeal margin with a broad anteclypeal apron or flange that fits tightly over basal margins of mandibles and is at an angle to outline of clypeus proper (not a direct continuation of median clypeus). Anteclypeal apron of different sculpture/texture from median portion of clypeus." Within this tribe group there are two tribes: Attini (the fungus-growing ants) and Blepharidattini. Workers of Attini are differentiated from the Blepharidattini by (1) the fungus-growing habit; (2) masticatory margin of mandibles longer than basal margin, usually with 7 or more teeth (secondarily reduced to 5 teeth in some groups); and (3) the 11-segmented antennae gradually incrassate. In contrast, the Blepharidattini (1) do not culture fungi; (2) have relatively shorter mandibles, the masticatory margin with 5 or fewer teeth and subequal in length to the basal margin; and (3) the 11-segmented antennae have a discrete 2-segmented club. Additional blepharidattine characters described by Bolton are clypeus broadly inserted between the frontal lobes and the propodeal spiracle low on the side of the propodeum. The Blepharidattini contains two genera, Blepharidatta and Wasmannia. Blepharidatta have very strongly developed antennal scrobes, the upper margin of which is prolonged posteriorly so that the posterolateral margins of the vertex are drawn out as posteriorly directed teeth or lobes, the anterior margins of the frontal lobes project forward almost to the anterior margin of the clypeus, and the petiolar node is long, low, and without differentiated anterior face. In Wasmannia, the antennal scrobes are shallow and never so strongly developed; the posterolateral margins of the vertex are not produced as lobes or teeth; the frontal lobes do not project forward; and the petiolar node is always well-developed, with a distinct anterior face.
In practice, Wasmannia may be confused with some species of Ochetomyrmex. For example, the face of O. semipolita is very similar to Wasmannia (Fernandez 2003). Ochetomyrmex have less developed antennal scrobes, the clypeal apron is lacking, and there is a slightly impressed mesonotal suture which is never present in Wasmannia. In addition, Wasmannia lacks a bifurcated carina on the ventral surface of the petiole, a character present in Ochetomyrmex, Tranopelta, and other myrmicines (Fernandez 2003).
The genus was recently reviewed by Longino and Fernandez (2007).
The following terminology and abbreviations are used in the key:
HL: head length; perpendicular distance from line tangent to rearmost points of vertex margin to line tangent to anteriormost projections of clypeus, including anterior flange, in full face view.
HW: head width; maximum width of head in face view, including eyes if they project beyond the sides of the head.
EL: eye length, measured along maximum diameter.
WL: Weber's length; viewing mesosoma in lateral profile, distance from approximate inflection point, where downward sloping pronotum curves into anteriorly projecting neck, to posteroventral propodeal lobes.
CI: cephalic index; HW/HL.
OI: ocular index; EL/HL.
Measurements for Wasmannia specimens are reported in Table 1 below.
Kusnezov (1952) observed that Wasmannia auropunctata was the dominant Wasmannia species in Argentina and that it had the greatest difference between worker size and queen size. The Argentinean species sulcaticeps and williamsoni were restricted to the subtropical and semiarid limits of the genus, and the queens were much smaller and closer to the workers in size. He suggested that the species such as sulcaticeps and williamsoni were the more primitive members of the genus, and auropunctata was more recent and more advanced. We examined the relationship between worker size and queen size for the eight species of Wasmannia for which we had data, and there is a negative relationship (Fig. 1). Species with the smallest workers have the largest queens. The species fall roughly into three groups. Wasmannia sigmoidea, iheringi, sulcaticeps, and scrobifera have very small queens, little larger than the workers. Wasmannia rochai, affinis, and lutzi are intermediate, with relatively smaller workers and larger queens. Wasmannia auropunctata is at the top, with the smallest workers and largest queens.
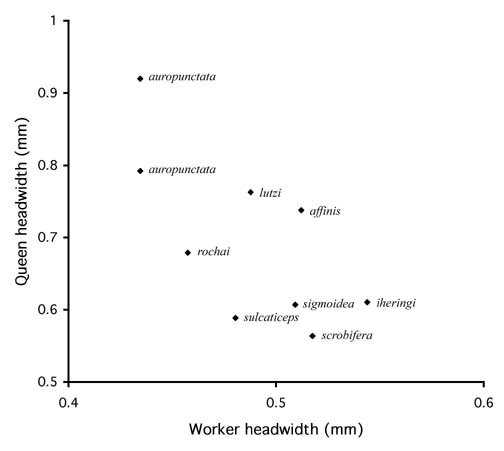
Figure 1. Relationship of queen head width to worker head width among Wasmannia species. Average values are shown, based on data in Table 1. Wasmannia auropunctata is plotted twice, with different values for the dimorphic queens. A regression of queen head width on worker head width has a negative slope, r2=0.50, p<0.05 (removing the large-headed W. auropunctata queens renders the regression nonsignificant).
These results support Kusnezov's conclusions. A general trend in ant evolution is the development of increasing caste differences, and it follows that greater differences in size between worker and queen is often apomorphic. If that is the case with Wasmannia, then auropunctata, lutzi, affinis, and rochai may form a clade within Wasmannia based on larger queen size. If increasing caste differentiation is associated with greater ecological success, the ecological dominance of auropunctata could be explained by it having the largest queens and smallest workers in the genus.
A case can also be made for iheringi being the sister taxon to all other Wasmannia. It has the most plesiomorphic mesosoma and petiole shape, resembling other generalized myrmicines. Other Wasmannia show a trend toward shorter, more compact mesosoma and shorter petiolar peduncle and/or more quadrate node. A biogeographic scenario would thus have iheringi as the oldest lineage in the genus, with disjunct populations at scattered localities over a large area, having been largely displaced from a formerly extensive range. Other rare or localized species in the genus — sulcaticeps, williamsoni, sigmoidea, scrobifera, affinis, lutzi — could also be relicts from an early radiation of the genus. Wasmannia rochai is the second most common and widespread species. Its small workers and large queens could have been an initial step in the direction of ecological dominance. Wasmannia auropunctata, with its even smaller workers and larger queens, is the most recent manifestation of this trend. It has swept the Neotropical field and is now conquering the world.
Literature Cited
Bolton, B. 2003. Synopsis and classification of Formicidae. Memoirs of the American Entomological Institute 71:1-370.
Fernández, F. 2003. The myrmicine ant genera Ochetomyrmex Mayr and Tranopelta Mayr (Hymenoptera: Formicidae). Sociobiology 41:633-661.
Forel, A. 1893. Formicides de l'Antille St. Vincent, récoltées par Mons. H. H. Smith. Transactions of the Entomological Society of London 1893:333-418.
Kusnezov, N. 1952 ("1951"). El género Wasmannia en la Argentina (Hymenoptera, Formicidae). Acta Zoologica Lilloana 10:173-182.
Longino, J. T., and F. Fernández C. 2007. A taxonomic review of the genus Wasmannia. Pages 271-289 in R. R. Snelling, B. L. Fisher, and P. S. Ward, editors. Advances In Ant Systematics (Hymenoptera: Formicidae): Homage To E. O. Wilson – 50 Years Of Contributions. Memoirs of the American Entomological Institute, 80.
Table 1. Measurement data for Wasmannia queens and workers.
species barcode caste WL HW (max width including eyes) HL EL CI OI
affinis holotype w 0.581 0.5143 0.5531 0.113 0.929849937 0.204303019
affinis Fernando specimen q 1.16 0.74 0.72 0.16 1.01 0.22
auropunctata obscura lectotype w 0.4591 0.4204 0.4572 0.1067 0.919510061 0.233377078
auropunctata rugosa syntype w 0.4826 0.4489 0.475 0.0965 0.945052632 0.203157895
auropunctata INBIOCRI002280111 q 0.7506 0.6737 1.114145762
auropunctata INBIOCRI002280087 q 1.1259 0.7734 0.6852 0.2229 1.128721541 0.32530648
auropunctata INBIOCRI002280100 q 0.7703 0.6877 1.120110513
auropunctata INBIOCRI002280092 q 0.7671 0.6953 1.103264778
auropunctata INBIOCRI001283246 q 0.7855 0.6979 1.125519415
auropunctata INBIOCRI002280107 q 1.19 0.8014 0.7099 0.2134 1.128891393 0.300605719
auropunctata INBIOCRI002280095 q 0.7772 0.7233 1.074519563
auropunctata INBIOCRI002280098 q 0.8007 0.7283 1.099409584
auropunctata INBIOCRI001282859 q 0.816 0.7315 1.115516063
auropunctata JTLC000003405 q 0.8388 0.7385 1.135815843
auropunctata JTLC000003408 q 0.8172 0.748 1.092513369
auropunctata INBIOCRI001283243 q 0.809 0.7569 1.068833399
auropunctata INBIOCRI002280099 q 0.9004 0.835 1.078323353
auropunctata INBIOCRI002280093 q 0.9119 0.8369 1.089616442
auropunctata INBIOCRI002280097 q 0.9188 0.8515 1.079036994
auropunctata INBIOCRI001237395 q 0.9493 0.8636 1.099235757
auropunctata holotype glabra q 1.3341 0.8439 0.7436 0.2616 1.134884346 0.351802044
iheringi syntype w 0.6521 0.5651 0.5315 0.1111 1.06321731 0.209031044
iheringi INBIOCRI001271987 w 0.6458 0.5226 0.5378 0.1289 0.971736705 0.239680179
iheringi INBIOCRI001271987 q 0.8592 0.5994 0.6064 0.1753 0.988456464 0.289083113
iheringi syntype q 0.875 0.6217 0.6267 0.1638 0.992021701 0.261369076
lutzi syntype w 0.5645 0.4877 0.5467 0.1022 0.892079751 0.186939821
lutzi syntype q 1.1532 0.7626 0.6909 0.1899 1.103777681 0.27485888
rochai LACM ENT 140078 w 0.4572 0.4299 0.4451 0.1054 0.965850371 0.236800719
rochai LACM ENT 141402 w 0.4477 0.4432 0.4635 0.0978 0.956202805 0.211003236
rochai lectotype w 0.5397 0.4991 0.5029 0.1118 0.992443826 0.222310599
rochai LACM ENT 141402 q 1.077 0.6769 0.6394 0.2197 1.058648733 0.343603378
rochai INBIOCRI002280101 q 1.077 0.6814 0.6496 0.1994 1.048953202 0.306958128
scrobifera INBIOCRI002280114 w 0.5645 0.5175 0.522 0.1524 0.99137931 0.291954023
scrobifera INBIOCRI002280114 q 0.6712 0.5639 0.5988 0.1702 0.941716767 0.284235137
sigmoidea LACM ENT 141401 w 0.5626 0.4877 0.5474 0.1441 0.890938984 0.263244428
sigmoidea lectotype w 0.5778 0.5086 0.5569 0.1441 0.913269887 0.258753816
sigmoidea INB0003235900 w 0.6045 0.5169 0.574 0.1473 0.900522648 0.256620209
sigmoidea INB0003231652 w 0.6071 0.5239 0.5817 0.1511 0.900636067 0.259755888
sigmoidea st.vincent Forel q 0.8204 0.6071 0.6242 0.2038 0.972604934 0.326497917
sulcaticeps syntype bruchi w 0.5696 0.4832 0.5467 0.1219 0.883848546 0.222974209
sulcaticeps syntype weiseri, top queen q 0.802 0.5918 0.6331 0.1759 0.93476544 0.277839204
williamsoni Kusnezov 6060 w 0.71 0.59 0.69 0.15 0.85 0.22
Page authors:
John T. Longino, The Evergreen State College, Olympia WA 98505
USA. longinoj@evergreen.edu
Fernando Fernández, Instituto de Ciencias Naturales,
Universidad Nacional de Colombia,
Apartado 7495,
Bogotá D.C.,
COLOMBIA
Date of this version: 26 September 2007.
Go to Ants of Costa Rica Homepage
