Myrmicinae, Formicidae, Hymenoptera, Insecta, Arthropoda, Animalia
Additional images: Queen from La Selva, face view (small, large), lateral view (small, large), petiole (small, large).
Queen from PeĖas Blancas, lateral view (small, large).
Aberrant worker with swollen head, face view (small, large), lateral view (small, large).
Wasmannia auropunctata atomum: syntype
Wasmannia auropunctata glabra: holotype
Wasmannia auropunctata obscura: lectotype
Wasmannia auropunctata pulla: syntype
Wasmannia auropunctata rugosa: syntype
Range
Native to the Neotropics, widespread in tropical America from Florida to Argentina, often an exotic pest ant elsewhere in the tropics and subtropics, especially on islands.
Identification
The strongly quadrate lateral profile of the petiolar node is unique to this species.
Natural History
Wasmannia auropunctata is a widespread pest ant (Clark et al. 1982, De Souza et al 1998, Fabres and Brown 1978, Jourdan 1997, Longino and Fernández 2007, Lubin 1984, Ulloa Chacón and Cherix 1990, Williams 1994, Wetterer and Porter 2003). In its presumed native range it occurs from Argentina to Mexico (Kempf 1972, Wetterer and Porter 2003). Its introduced range includes the Galapagos Islands, West Africa (Gabon, Cameroon, and possibly the Republic of Congo and the Democratic RepubIic of Congo), Melanesia (New Caledonia, Solomon Islands, Vanuatu, and possibly Tuvalu), Polynesia (Wallis and Futuna and Hawaii), parts of the US (Florida and possibly California), and subtropical Atlantic islands (the Bahamas and Bermuda) (Wetterer and Porter 2003). It is widespread on Caribbean islands, but it is unclear whether these are long-term native populations or recent introductions (Wetterer and Porter 2003).
The species is remarkably catholic in its habitat preference. It is common in habitats ranging from wet to dry and from early successional to mature. In an elevational gradient of mature wet forest on the Atlantic slope of Costa Rica (the Barva Transect, from La Selva Biological Station to 2000m elevation on the slope of Volcan Barva) it is abundant at 50m and 500m elevations, but nearly absent at 1070m. In the lowland habitats where it is abundant, it occurs in leaf litter on the forest floor and at all levels in the vegetation.
Although it occurs frequently in samples from mature forest habitats in Costa Rica, it is never so abundant in those habitats that it is noticeable as a pest or appears to be displacing other native species (Tennant 1994, McGlynn and Kirksey 2000, pers. obs.). In contrast, in certain agricultural habitats (banana plantations) and in parts of the tropics where it has been introduced it becomes super-abundant, with negative impacts on native species and human comfort (Clark et al. 1982, Wetterer and Porter 2003). In dry-forest fragments in Colombia there is a negative correlation between W. auropunctata abundance and overall ant diversity (Armbrecht and Ulloa Chacón 2003). Where introduced in New Caledonia it invades dense native forest and displaces native ants (Le Breton et al. 2003). Behavioral tests and cuticular hydrocarbon analysis show that W. auropunctata is multicolonial in its native range in Brazil, unicolonial where introduced in New Caledonia (Errard et al. 2002).
The sting of W. auropunctata is noteworthy. These are extremely tiny ants, barely visible in the field. When the senior author first began studying ants in Costa Rica, he was at first puzzled about Wasmannia. By literature accounts Wasmannia was reputed to have a terrible sting, but he had been collecting them for months in Corcovado National Park without ever experiencing the famous sting. One day he was collecting from a populous nest and some workers made it up to the soft skin of his inner forearm and began to sting. The sting was definitely noticeable, about as severe as a fire ant (i.e., Solenopsis geminata) but inordinately strong for an ant that could barely be seen! Workers are so small they cannot sting through the thicker skin of the hands.
Surprisingly, the chemical and toxicological nature of the venom of W. auropunctata has not been investigated. Howard et al. (1982) discovered an alkylpyrazine compound in the mandibular glands, which acted as an attractant to conspecifics and a repellent to heterospecifics. They speculated that the workers might apply the mandibular gland product as an irritating secretion, augmenting the defensive properties of the venomous sting. It would be interesting to investigate whether the venom alone is the powerful agent in this small ant, or if the strong burning sensation is a synergetic effect of venom plus mandibular gland product.
Nests can be almost anywhere: in rolled leaves or dead sticks in the leaf litter, under stones, in rotten wood, in hollow stems suspended above the ground, in ant-plant domatia, and under epiphytes. Workers are omnivorous scavengers and predators and can rapidly recruit to food. Colonies are polygynous and it is never clear where colony boundaries are. Dozens of dealate queens may be found together in nests. Males are rare but do occasionally occur.
Fournier et al. (2005) discovered that W. auropunctata has remarkable and peculiar reproductive biology. Their abstract is as follows:
Sexual reproduction can lead to major conflicts between sexes and within genomes. Here we report an extreme case of such conflicts in the little fire ant Wasmannia auropunctata. We found that sterile workers are produced by normal sexual reproduction, whereas daughter queens are invariably clonally produced. Because males usually develop from unfertilized maternal eggs in ants and other haplodiploid species, they normally achieve direct fitness only through diploid female offspring. Hence, although the clonal production of queens increases the queen's relatedness to reproductive daughters, it potentially reduces male reproductive success to zero. In an apparent response to this conflict between sexes, genetic analyses reveal that males reproduce clonally, most likely by eliminating the maternal half of the genome in diploid eggs. As a result, all sons have nuclear genomes identical to those of their father. The obligate clonal production of males and queens from individuals of the same sex effectively results in a complete separation of the male and female gene pools. These findings show that the haplodiploid sex-determination system provides grounds for the evolution of extraordinary genetic systems and new types of sexual conflict.
Comments
Workers of W. auropunctata have a strongly quadrate petiolar node. The anterior face of the node is sharply differentiated from both the peduncle and the dorsal face of the node, meeting both at nearly right angles, and forming a strongly step-like profile. This is a highly distinctive feature that easily distinguishes auropunctata workers from all other Wasmannia species. Within the species there is abundant variation in the strength of sculpturing and coloration, and this has engendered the naming of nine infraspecific forms in addition to the nominotypical, all of which are now junior synonyms (Longino and Fernandez 2007).
The abundant intraspecific variation suggests genetic structuring, and perhaps species-level differences, within the broad concept of auropunctata. There may even be distinct sympatric species. In Costa Rica, there appear to be two size classes of queens (Fig. 1). Queens with smaller heads include ten from various sites in Costa Rica, including La Selva Biological Station and the PeĖas Blancas Valley, one from Jamaica, and one from Venezuela. Queens with large heads are all from the Atlantic slope of Costa Rica. Three are from La Selva (two from different Winkler samples of sifted leaf litter from the forest floor, one from a small nest under an epiphyte mat in an old treefall) and one is an alate queen found in a Cecropia sapling near Volcan Arenal. Thus the small-headed and big-headed forms are broadly sympatric in Costa Rica. Among the small-headed queens four are definitively associated with workers from the same colony, and among the big-headed queens one is associated with workers. Others have workers doubtfully associated (together in the same Winkler sample). We can discern no differences in workers associated with the two types of queens. The cause of the two size classes of queens is unknown, but could reflect either differences between cryptic species or intraspecific polymorphism.
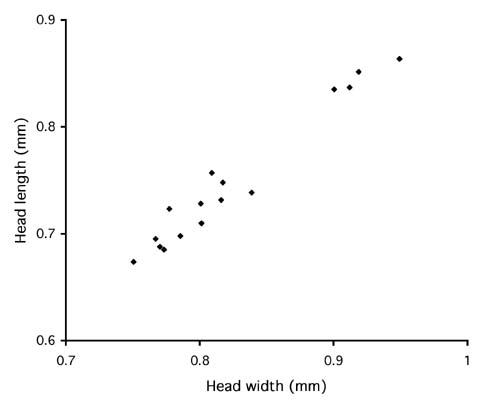
Figure 1. Queens of Wasmannia auropunctata occur in two size classes. In cluster of queens with smaller head size, one is from Jamaica and one from Venezuela. All others (n=14) are from Costa Rica.
An aberrant worker form is frequently encountered in large samples of W. auropunctata. These aberrant workers have the head grossly swollen. The entire head is more spherical than normal, as if the head were inflated like a balloon. The rest of the body is little different from a normal worker. These aberrant workers are occasionally encountered in Winkler samples that contain hundreds or thousands of auropunctata workers. (Aberrant worker with swollen head, face view [small, large], lateral view [small, large].)
Literature Cited
Armbrecht, I., and P. Ulloa-Chacon. 2003. The little fire ant Wasmannia auropunctata (Roger) (Hymenoptera : Formicidae) as a diversity indicator of ants in tropical dry forest fragments of Colombia. Environmental Entomology 32:542-547.
Clark, D. B., C. Guayasamín, O. PazmiĖo, C. Donoso, and Y. Páez de Villacís. 1982. The tramp ant Wasmannia auropunctata: autecology and effects on ant diversity and distribution on Santa Cruz Island, Galapagos. Biotropica 14:196-207.
De Souza, A. L. B., J. H. C. Delabie, and H. G. Fowler. 1998. Wasmannia spp. (Hym. Formicidae) and insect damages to cocoa in Brazilian farms. Journal of Applied Entomology 122:339-341.
Errard, C., H. Jourdan, C. Glaude, J. H. Delabie, and A. Hefetz. 2002. Intraspecific and interspecific discrimination in the tramp ant Wasmannia auropunctata (Hym. Formicidae). Actes des Colloques Insectes Sociaux 15:82-89.
Fabres, G., and W. L. Brown, Jr. 1978. The recent introduction of the pest ant Wasmannia auropunctata into New Caledonia. Journal of the Australian Entomological Society 17:139-142.
Fournier, D., A. Estoup, R. M. Orivel, J. Foucaud, H. Jourdan, J. Le Breton, and L. Keller. 2005. Clonal reproduction by males and females in the little fire ant. Nature 435:1230-1234.
Howard, D. F., M. S. Blum, T. H. Jones & Tomalski, M. D. 1982. Behavioral responses to an alkylpyrazine from the mandibular gland of the ant Wasmannia auropunctata. Insectes Sociaux 29:369-374.
Jourdan, H. 1997. Threats on Pacific islands: the spread of the tramp ant Wasmannia auropunctata (Hymenoptera: Formicidae). Pac. Cons. Biol. 3:61-64.
Kempf, W. W. 1972. Catálogo abreviado das formigas da Regičo Neotropical. Studia Entomologica 15:3-344.
Le Breton, J., J. Chazeau, and H. Jourdan. 2003. Immediate impacts of invasion by Wasmannia auropunctata (Hymenoptera : Formicidae) on native litter ant fauna in a New Caledonian rainforest. Austral Ecology 28:204-209.
Longino, J. T., and F. Fernández C. 2007. A taxonomic review of the genus Wasmannia. Pages 271-289 in R. R. Snelling, B. L. Fisher, and P. S. Ward, editors. Advances In Ant Systematics (Hymenoptera: Formicidae): Homage To E. O. Wilson – 50 Years Of Contributions. Memoirs of the American Entomological Institute, 80.
Lubin, Y. D. 1984. Changes in the native fauna of the Galápagos Islands following invasion by the little red fire ant, Wasmannia auropunctata. Biological Journal of the Linnean Society 21:229-242.
McGlynn, T. P., and S. E. Kirksey. 2000. The effects of food presentation and microhabitat upon resource monopoly in a ground-foraging ant (Hymenoptera: Formicidae) community. Revista de Biologia Tropical 48:629-642.
Tennant, L. E. 1994. The ecology of Wasmannia auropunctata in primary tropical rainforest in Costa Rica and Panama. Pp. 80-90 in: Williams, D. F. (ed). Exotic ants: biology, impact, and control of introduced species: 332 pp. Westview Press, Boulder, Colorado, USA.
Ulloa Chacón, D., and D. Cherix. 1990. The little fire ant Wasmannia auropunctata (Roger)(Hymenoptera: Formicidae). Pages 281-289 in R. K. Vander Meer, K. Jaffe, and A. Cedeno, editors. Applied myrmecology: a world perspective. Westview press, Boulder, CO. 741 p.
Wetterer, J. K., and S. D. Porter. 2003. The little fire ant, Wasmannia auropunctata: distribution, impact, and control. Sociobiology 42:1-41.
Williams, D. F., (ed.). 1994. Exotic ants. Biology, impact, and control of introduced species. Westview Press, Boulder. [Numerous articles in this book concern the biology of Wasmannia auropunctata.]
Page authors:
John T. Longino, The Evergreen State College, Olympia WA 98505 USA. longinoj@evergreen.edu
Fernando Fernández, Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado 7495, Bogotá D.C., COLOMBIA