Myrmicinae, Formicidae, Hymenoptera, Insecta, Arthropoda, Animalia
Images above are of holotype. Additional images of holotype: worker dorsal view (large); worker petiole, lateral view (large).
Range
Costa Rica: Atlantic slope wet forest from 50m to 1600m.
Identification
Basal margin of mandible flat, without a notch; face with a distinct fan of longitudinal rugae between frontal lobes, and rugose sculpture that extends from mandibular insertions to beyond compound eye; rest of face and promesonotum smooth and highly polished; propodeum shiny and polished like promesonotum, but with variably developed transverse rugae on dorsal face; legs red brown, contrasting with darker mesosoma.
Variation: There is a tendency for workers to be smaller and more strongly sculptured at lower elevation. However, an isolated worker from the 2000m site, tentatively identified as this species, is aberrant in several respects. It is relatively large. The facial sculpture is more extensive than on any other specimens, extending over about 3/4's of the face, leaving a narrow posterior strip that is smooth and polished. The pronotum, instead of being completely smooth, has feeble, thin, widely spaced, transverse striae. A specimen from Panama, Bocas del Toro, Fortuna-Chiriqui Grande road, 800m (D. Olson) has similar face sculpture but even heavier sculpture, with abundant transverse striae on the promesonotum and dorsal face of propodeum. However, it is smaller and with light-colored legs, like the typical form. This specimen is so different I have given it a different morphospecies code: JTL-014.
Similar species: Stenamma expolitum.
Natural History
Stenamma alas occurs in mature wet forest habitats. It is a specialist inhabitant of clay banks. Colonies are most readily encountered on nearly vertical banks along stream margins, but may also occur along trail edges where they have been cut into hillsides.
This and the related species S. expolitum have complex nesting behavior, reported in Longino (2005). The text from that article (with a few modifications) follows:
Two Neotropical ant species, Stenamma expolitum Smith and S. alas Longino, exhibit three unusual nesting behaviors: (1) they build architecturally sophisticated nest entrances that elevate the nest opening away from the surface; (2) they maintain multiple identical nests but occupy only one of them; and (3) they keep a round "door pebble" at the nest entrance, with which they plug the opening in response to army ants. Adaptive hypotheses for these behaviors are discussed, including the possibility that they are multiple lines of defense against army ant predation.
Introduction
Tropical rainforests are laced with a skein of ants that influence many ecological and evolutionary processes. Nest construction is a major aspect of the natural history of rainforest ants, and the majority of ant species can be classified into a few general categories of nesting behavior (Wheeler 1910, Forel 1928). The classic ant nest is a subterranean chamber with an entrance tunnel and a tumulus of soil on the surface. Others have small nests in the leaf litter, nesting in chambers in small twigs, in dead seeds, or between rotting leaves (Byrne 1994, Kaspari 1996). Arboreal ants nest in dead or live branches in the canopy, and some make exposed carton nests. Several groups of ants have very specialized nesting habits that have attracted the attention of naturalists. Army ants have nomadic behavior, building temporary bivouacs composed of their own bodies (Schnierla 1971, Gotwald 1995). Specialized plant ants have mutualistic relationships with plants, nesting in preformed chambers unique to particular myrmecophyte taxa (Bequaert 1922). These specialized nesting behaviors have attracted attention both for their inherent interest as unique phenomena and for what they reveal about the ecology and evolution of rainforest ants.
I report here the novel nesting behavior of two species of Neotropical ants. The exceptional nature of their nesting behavior will place them in the pool of tropical oddities that attract inherent interest. However, studies of the functions of the behavior may help reveal the major selection pressures that shape ant communities in general. Preliminary observations reported here suggest a major role for army ants.
Methods
Geographic range data are based on museum collections. More detailed behavioral observations were made on 28 colonies at four Costa Rican localities: (1) Alajuela Province, R’o Pe–as Blancas, 800m elevation, 10ˇ19'N 84ˇ43'W, February 2004; (2) Heredia Province, Braulio Carrillo National Park, Barva transect, 500m elevation, 10ˇ20'N 84ˇ05'W, February 2003; (3) Barva transect, 300m elevation, 10ˇ21'N 84ˇ03'W, March 2004; and (4) La Selva Biological Station, 50m elevation, 10ˇ26'N 84ˇ01'W, June and August 2004. All localities are mature rainforest sites on the Atlantic slope. The majority of observations are from the Barva transect in Braulio Carrillo National Park, a protected zone from the top of Volcan Barva to La Selva Biological Station (Hartshorn and Peralta 1988, Lieberman et al. 1996, McDade et al. 1994, Pringle et al. 1984). All are very humid sites, with annual rainfall from 4 to 7m, depending on elevation. Nests were carefully examined and the architecture of the nest entrance recorded. At least one voucher specimen from each nest was collected. Vouchers are currently in the author's collection but will ultimately be deposited in the Instituto Nacional de Biodiversidad (Costa Rica) and other major research collections. A subset of the nests was subjected to a series of behavioral trials over a 1-3 day period, the details of which are described below. Some nests were thoroughly excavated so that the entire nest structure could be seen and the entire colony population counted.
Results
Geographic range, abundance, and habitat preference. -- Stenamma expolitum and S. alas are both endemic to the Atlantic slope of Costa Rica. Stenamma expolitum was described in 1962, based on specimens from Colombiana Farm, a site in Lim—n Province near Turrialba. Recent collections have been from the Monteverde and upper Pe–as Blancas area of the Cordillera de Tilar‡n, and from the Barva transect. On the Barva transect, one or both occur from 50m elevation at La Selva to 1600m elevation.
Both species are specialist inhabitants of nearly vertical clay banks in mature forest habitats. Until recently they were considered extremely rare ants. The Monteverde and Pe–as Blancas area in the Cordillera de Tilar‡n and the Barva Transect, including La Selva Biological Station, have been abundantly surveyed for ants using Winkler and Berlese samples of leaf litter from the forest floor (Longino and Colwell 1997, Longino et al. 2002, Longino and Nadkarni 1990, Schonberg and Longino unpub.). These programs of quantitative sampling have yielded one S. expolitum worker from La Selva, and workers of S. alas from six Winkler samples from Monteverde. Neither species has ever been collected at baits or with Malaise traps, pan traps, or canopy fogging. In contrast, directed search along clay banks in Pe–as Blancas, the Barva Transect, and La Selva quickly yielded multiple nest samples of both species.
At the Cantarrana site, at 300m elevation on the Barva Transect, a careful search along the banks of a stream yielded 12 colonies of S. expolitum and nine colonies of S. alas, along 0.5km of stream. Colonies were predictably present on segments of steeply inclined to concave clay bank at the stream edge. These banks were 2-5m tall, usually with a sharply delimited upper edge where forest floor leaf litter and overhanging plant roots began. A temporal succession of clay banks could be seen, beginning with recently exposed surfaces, then a sparse covering of small bryophytes and other small plants, and finally a dense covering of bryophytes. Ants were absent from the new banks, very abundant on banks of intermediate stage, and much less abundant on the moss-covered banks. The clay was stiff, so it could be cut with a knife and hold its shape. Banks where the soil was more weathered and friable did not have Stenamma nests. Casual inspection of vertical banks along road cuts and trails also revealed Stenamma nests. Areas where Stenamma were found were always heavily shaded, either in mature forest or well-developed second growth forest.
Decades of work on ants at La Selva, including an intensive ant inventory (Longino et al. 2002), failed to record either of these Stenamma species. Yet knowledge of the specialized microhabitat allowed the recent location of two nests of S. expolitum and one nest of S. alas within one hour of searching.
Nest structure, multiple nests, and colony size. -- The nest of S. alas is comprised of an alcove in the bank, within which is a vertical disk of accreted soil, raised on a narrow stem (Fig. 1). This pedestal-like structure is the nest entrance, and a tunnel extends horizontally from the center of the disk, through the stem, and back into the bank. The entrance tunnel expands into a flat chamber that is about 8cm deep, 2cm wide, and 5mm high. The nest of S. expolitum is in a similar alcove, within which is a mound of spherical pebbles (Fig. 1). A circular disk of accreted soil, similar to that of S. alas, is variably present on top of the mound, but is horizontal rather than vertical. The entrance tunnel descends vertically through the mound and then curves back into the bank, where there is a chamber identical to that seen in S. alas. In addition, there is a second, smaller chamber that extends into the bank from the back of the alcove, behind the mound. There are no internal connections between the two chambers. In both species, recent excavation activity is visible as a conspicuous ring of accreted soil particles on the bank, encircling the alcove (Fig. 1).
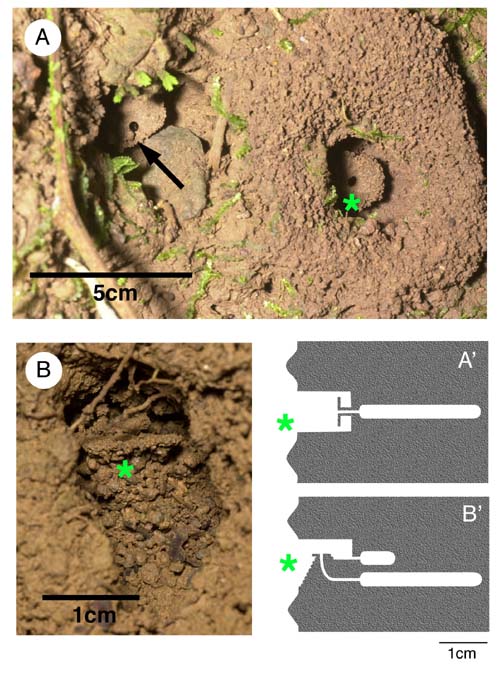
Figure 1. Nest architecture of two species of the ant genus Stenamma. Nests are in vertical clay banks in wet forest habitats. A. Two nest entrances of Stenamma alas. Arrow points to door pebble of active nest; more conspicuous nest to right shows active excavation but is unoccupied. A'. Schematic lateral view of nest architecture. B. Nest entrance of S. expolitum. Note horizontal disk, nest entrance, and door pebble at apex of pebble mound. B'. Schematic lateral view of nest architecture. Asterisks coordinate anterior and lateral views of nests.
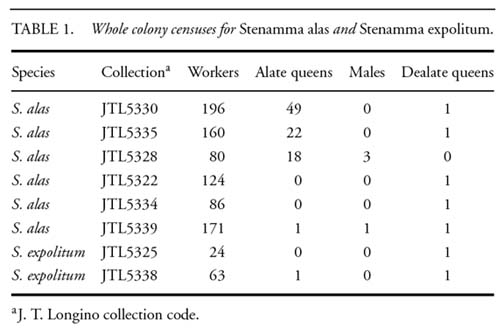
For both species, mature colonies maintain two or three nests in close proximity. Distances between pairs of nearest-neighbor nests averaged 18.9cm (SE 2.3cm, n=27). Colonies are separated by much larger distances, usually on different patches of suitable clay bank. The intra-colonial nature of the nest clusters is obvious. Only one of the nests is inhabited and the others are either empty or contain one to three workers. The occupied nest contains up to 200 workers, a single colony queen, and brood in variable stages of development (Table 1). For S. expolitum, the upper chamber usually contains about ten workers, with the remainder of the colony in the large lower chamber. When a fresh ring of excavated soil is present it is around one of the uninhabited nests. When I artificially plugged nest entrances, workers quickly removed plugs (within minutes) from the occupied nest and at least one of the unoccupied nests. This shows that the multiple nests are actively maintained and do not represent a series of abandoned nests.
Door pellet and response to disturbance. -- Both species maintain a spherical pebble near the entrance. For S. alas the pebble adheres to the vertical clay disk just below the entrance. For S. expolitum the pebble perches at the top of the mound or on the horizontal clay disk when the disk is present. When the pebble is removed, workers will retrieve it or replace it within minutes. I observed the pebble being used to close the nest entrance (Fig. 2) in response to army ants. I performed trials by grasping army ant workers with forceps and holding them near nest entrances. I used live army ant workers from raiding columns of Eciton mexicanum and Neivamyrmex gibbatus. In 11 of 13 trials in which the only treatment was to present an army ant worker, a Stenamma worker from inside the nest immediately grabbed the pebble and pulled it into the nest entrance. The pebble filled the nest entrance like a stopper. In the case of S. expolitum, the workers in the upper chamber were left stranded outside the main nest chamber. In five additional trials I first tapped the nest entrance with forceps, in some cases even breaking a portion of the disk, and then followed with the presentation of an army ant. Stenamma workers never attempted to plug the entrance following the mechanical disturbance, but in all five cases immediately closed the nest on encounter with the army ant. In two further trials I first presented a different ant species (one trial with a worker of Pheidole simonsi and one with Aphaenogaster araneoides). In neither case did the Stenamma close the nest. When followed by the presentation of an army ant, they immediately closed the nest. Thus neither mechanical disturbance of the nest entrance nor presentation of other non-ecitonine ant workers elicits nest closure.

Figure 2. Stenamma expolitum using door pellet to close entrance.
Some species of ants exhibit panic evacuations in response to army ant attack (LaMon & Topoff 1981, Droual 1981). Workers from a besieged colony, each bearing a larva, pour forth from the nest entrance and scatter, slowly returning to the nest after the raid has passed. During excavations of Stenamma nests and presentations of army ants I never observed evacuations. The one immigration I observed was at 8:30am, and there was no sign of attack or trauma.
Discussion
The three behaviors I have described Ń elevated nest entrances, multiple empty nests, and door closure Ń remain to be investigated for functional significance. Architecturally complex nest entrances are known for a few species of ants (Forel 1928). The Asian ant Harpegnathos saltator builds a complex subterranean nest in which a simple entrance leads to an antechamber, and a molded funnel-like superstructure projects from the main nest chamber into the antechamber (Peeters et al. 1994). Peeters et al. suggested the adaptive significance of the elevated nest entrance was to prevent flooding in the monsoonal climate. Crosland (1995) observed a related nest structure in H. venator. This species nests in clay banks and has both an antechamber and main nest chamber, like H. saltator. Both the outer nest entrance on the clay bank and the inner opening between the main chamber and antechamber are surmounted by a funnel-like superstructure. Crosland suggested that the funnel-like nest entrance might function in predator evasion.
Three hypotheses can be proposed to explain the complex and elevated nest entrances of S. alas and expolitum. One is that the elevated entrances reduce the likelihood of flooding. Rainfall is high in the habitats where these species live, and sheet flow over the clay surface may be common. However, a simple entrance with a downward slope would achieve the same end. A second hypothesis, suggested by M. Kaspari (pers. comm.) is that the nest entrances are related to sexual selection and mate choice. These two species are very closely related, yet their nest entrances are highly distinctive and immediately distinguishable in the field. If mating occurs at or near the nest, searching males may use the nest entrances as visual cues for locating mates. A third hypothesis is that the structure reduces the odor signature of the colony, thus reducing the likelihood of army ant attack. Army ant workers are blind and it is surmised that at least column raiders use odor cues to locate prey (Rettenmeyer 1963, Schnierla 1971). The elevated superstructures of the Stenamma nest entrances may reduce the likelihood of physical contact by army ants and may better disperse colony odors.
Nest relocation by ants is a common phenomenon (Smallwood 1982, Banschbach and Herbers 1999), but usually in the context of colonies locating higher quality nest sites (Dornhaus et al. 2004) or being forced to move due to trauma (LaMon and Topoff 1981, Briese 1984). McGlynn et al. (2003, 2004) recently demonstrated a particularly "unprovoked" form of nest relocation in the rainforest ant Aphaenogaster araneoides. Colonies maintained multiple empty nests and frequently moved among them. In their study, moves between nests were not associated with trauma, attack, resource abundance, or competition from other colonies. McGlynn et al. hypothesized that regular nest relocations in A. araneoides were related to predator and/or parasite avoidance, and in particular suggested that relocations could prevent accumulation of colony odors that could attract army ants. My observations of Stenamma suggest a parallel phenomenon, and the same hypothesis can be applied.
Many ant species have some portion of the body, usually the head, modified to form a circular plug for the nest entrance (phragmosis, see Wheeler 1910, Brown 1968). Phragmosis has evolved multiple times independently, occurring in disparate ant lineages. There is obviously strong selection to evolve mechanisms to seal off nest entrances. The nest closing behavior of these Stenamma is a form of behavioral phragmosis, using a selected or shaped pebble rather than a body part. Although many phragmotic ants keep the nest entrance sealed except to allow workers in and out, these Stenamma close the nest entrance only when in contact with army ants. Highly selective nest closure in response to army ants has rarely been reported (LaMon and Topoff 1981) and the maintenance of a door pebble has never been reported. The additional chamber constructed by S. expolitum, with its small retinue of workers, may serve as a decoy during an attack or as an additional line of defense, leaving sacrificial workers outside to protect the sealed nest entrance.
Predation pressure by army ants (subfamily Ecitoninae) is a recurrent theme in the adaptive hypotheses proposed for the complex nesting behavior of Stenamma. Army ants are a set of species with large colonies and nomadic, group-raiding behavior. A large part of the army ant diet comprises the brood of other ant species, and army ant raids on other ant species are commonplace (Rettenmeyer 1963, Schnierla 1971, Gotwald 1995). It has been hypothesized that army ants regulate and maintain overall ant diversity by "cropping" abundant ant species, preventing competitive exclusion (Otis et al. 1986, Franks and Bossert 1983, Kaspari and O'Donnell 2003). Kaspari and O'Donnell have estimated that every square meter of rainforest floor may be visited nearly daily by army ants. It may be argued that the size differential between the Stenamma and the much larger Eciton workers used in some of the behavioral trials make the army ant defense hypotheses untenable. The entrances to the Stenamma nests are smaller than most Eciton workers. However, there are many species of Neivamyrmex that have uniformly small workers. Also, larger army ants in the genera Eciton, Labidus, and Nomamyrmex are strongly polymorphic, and many of the smaller workers are the same size as Stenamma. For example, I have seen Nomamyrmex esenbeckii, a species known for its large workers and propensity to attack Atta colonies (Swartz 1998), attacking a colony of Pheidole laticornis, a ground-nesting species with workers similar in size to Stenamma. Finally, army ants share certain semiochemicals because they respond to each others trail pheromones (Watkins 1964) and most New World army ants produce skatole, which gives them a characteristic foetid odor (Brown et al. 1979). Stenamma may react to a generalized army ant odor, rather than to the odors of particular species that are their most frequent predators.
Architecturally complex nest entrances are known for a few species of ants (Forel 1928, Peeters et al. 1994, Crosland 1995), movement among multiple nest sites is known for others (Smallwood 1982, Banschbach and Herbers 1999, Dornhaus et al. 2003, McGlynn et al. 2003, 2004), and some species of ants are known to block their nest entrances in response to disturbance (Wheeler 1910, Brown 1968, Marikovsky 1974, Swartz 1998). The occurrence of all three behaviors in the same species is unprecedented. If all of these behaviors are adaptations to avoid army ant predation, it suggests that even small ants with small colonies are subject to intense selection by army ants, and not just larger species with larger amounts of brood.
The specialized microhabitat of these ants and their distinctive nest entrances make them easy to locate and observe. They have the potential to be a model system, revealing the selective pressures shaping ant behavior and testing the emerging view that army ants are dominant forces in tropical ecosystems, driving evolution and structuring communities of rainforest ants.


Typical nest entrance.

Typical nest entrance. Note prominent door pebble.

Note multiple nests, one at upper right, one at lower right.
Both morphology and behavior suggest S. alas and S. expolitum together form a monophyletic group, with the highly polished surface sculpture and the elaborate nest entrance structure being synapomorphic. Within the group, S. expolitum exhibits a more derived condition in both of these characters. Stenamma alas retains a plesiomorphic patch of face sculpture, which is lost in S. expolitum. Stenamma expolitum also has the more derived nest structure, with pebble mound and second chamber.
In an email exchange, Arthur Goodman alerted me to another case in which a social insect uses prefabricated pellets to block entrances. The Malaysian termite Prohamitermes mirabilis has nests consisting of many cells with small round entrance holes connnecting them. The termites keep prefabricated pellets in the cells that exactly fit the entrance holes, and the number of pellets in the cells matches the number of entrance holes (Tho 1981). Tho suggested that the termites use the pellets to quickly seal entrance holes when the termites are under attack, but he did not directly observe this behavior. There is another article on the subject (Tho and Maschwitz 1988) but it is copyright protected and I do not have easy access to it.
Arthur Goodman also noted "Curiously, there seems to be a vertebrate parallel to this in the 'beaver baseballs' which are found in the burrows of mountain beaver (Aplodontia), but I'm not aware of a scientifically reliable account."
Bret Weinstein alerted me to the history of Capadoccia in modern Turkey. This area has underground cities that were carved out of rock by early inhabitants. They kept huge millstones perched at entrances, and when under seige they could descend underground and roll the millstones to close the door behind them. Perhaps a good common name for these entrance-blocking Stenamma would be "capadoccian ants."

Banschbach, V. S., and J. M. Herbers. 1999. Nest movements and population spatial structure of the forest ant Myrmica punctiventris (Hymenoptera: Formicidae). An. Ent. Soc. Amer. 92: 414-423.
Bequaert, J. 1922. Ants in their diverse relations to the plant world. Bul. Amer. Mus. Nat. Hist. 45: 333-584.
Briese, D. T. 1984. Interactions between a myrmecophagous ant and a prey species. J. Aust. Entomol. Soc. 23: 167-168.
Brown, C. A., J. F. Watkins, II , and D. W. Eldridge. 1979. Repression of bacteria and fungi by the army ant secretion: skatole. J. Kansas Entomol. Soc. 52: 119-122.
Brown, W. L., Jr. 1968 ("1967"). A new Pheidole with reversed phragmosis (Hymenoptera: Formicidae). Psyche (Cambridge) 74: 331-339.
Byrne, M. M. 1994. Ecology of twig-dwelling ants in a wet lowland tropical forest. Biotropica 26: 61-72.
Crosland, M. W. J. 1995. Nest and colony structure in the primitive ant, Harpegnathos venator (Smith) (Hymenoptera: Formicidae). Pan Pac. Entomol. 71: 18-23.
Dornhaus, A., N. R. Franks, R. M. Hawkins, and H. N. S. Shere. 2004. Ants move to improve Đ colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Animal Behav. 67: 959-963.
Droual, R. 1983. The organization of nest evacuation in Pheidole desertorum Wheeler and P. hyatti Emery (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 12: 203-208.
Forel, A. 1928. The social world of the ants compared with that of man. Volume 1. [Translated by C. K. Ogden.]. G. P. Putnam's Sons, London.
Franks, N. R., and W. H. Bossert. 1983. The influence of swarm raiding army ants on the patchiness and diversity of a tropical leaf litter ant community. In S. L. Sutton, T. C. Whitmore, and A. C. Chadwick (Eds). Tropical rain forest: ecology and management, pp 151-163. Special Publication of the British Ecological Society No. 2. Blackwell, Oxford.
Gotwald, W. H., Jr. 1995. Army ants: the biology of social predation. Cornell University Press, Ithaca, New York.
Hartshorn, G. S. and R. Peralta 1988. Preliminary description of primary forests along the La Selva-Volcan Barva altitudinal transect, Costa Rica. In F. Almeda and C. M. Pringle, (Eds.). Tropical rainforests: diversity and conservation, pp. 281-296. California Academy of Sciences, San Francisco, California.
Kaspari, M. 1996. Litter ant patchiness at the 1-m2 scale: disturbance dynamics in three Neotropical forests. Oecologia (Berlin) 107: 265-273.
Kaspari, M., and S. O'Donnell. 2003. High rates of army ant raids in the Neotropics and implications for ant colony and community structure. Evol. Ecol. Res. 5: 933-939.
LaMon, B., and H. Topoff. 1981. Avoiding predation by army ants: defensive behaviours of three ant species of the genus Camponotus. Animal Behav. 29: 1070-1081.
Lieberman, D., M. Lieberman, R. Peralta, and G. S. Hartshorn. 1996. Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. J. Ecol. 84: 137-152.
Longino, J. T. 2005. Complex nesting behavior by two neotropical species of the ant genus Stenamma (Hymenoptera: Formicidae). Biotropica 37:670-675.
Longino, J. T., and R. K. Colwell. 1997. Biodiversity assessment using structured inventory: capturing the ant fauna of a lowland tropical rainforest. Ecological Applications 7: 1263-1277.
Longino, J. T., R. K. Colwell, and J. A. Coddington. 2002. The ant fauna of a tropical rainforest: estimating species richness three different ways. Ecology 83: 689-702.
Longino, J. T., and N. M. Nadkarni. 1990. A comparison of ground and canopy leaf litter ants (Hymenoptera: Formicidae) in a neotropical montane forest. Psyche 97: 81-93.
Marikovsky, P. I. 1974. The biology of the ant Rossomyrmex proformicarum K. W. Arnoldi (1928). Insectes Sociaux 21: 301-308.
McDade, L. A., K. S. Bawa, H. A. Hespenheide, and G. S. Hartshorn (Eds.). 1994. La Selva, Ecology and Natural History of a Neotropical Rainforest. University of Chicago Press, Chicago, IL, USA.
McGlynn, T. P., R. A. Carr, J. H. Carson, and J. Buma. 2004. Frequent nest relocation in the ant Aphaenogaster araneoides: resources, competition, and natural enemies. Oikos 106:612-621.
McGlynn, T. P., M. D. Shotell, and M. S. Kelly. 2003. Responding to a variable environment: Home range, foraging behavior, and nest relocation in the Costa Rican rainforest ant Aphaenogaster araneoides. J. Insect Behav. 16: 687-701.
Otis, G. W., C. E. Santana, D. L. Crawford, and M. L. Higgins. 1986. The effect of foraging army ants on leaf-litter arthropods. Biotropica 18: 56-61.
Peeters, C., B. Hšlldobler, M. Moffett, and T. M. Musthak Ali. 1994. ''Wall-papering" and elaborate nest architecture in the ponerine ant Harpegnathos saltator. Insectes Sociaux 41: 211-218.
Pringle, C., I. Chacon, M. Grayum, H. Greene, G. Hartshorn, G. Schatz, G. Stiles, C. Gomez, and M. Rodriguez. 1984. Natural history observations and ecological evaluation of the La Selva Protection Zone, Costa Rica. Brenesia 22: 189-206.
Rettenmeyer, C. W. 1963. Behavioral studies of army ants. Univ. Kans. Sci. Bull. 44: 281-465.
Schneirla, T. C. 1971. Army ants. A study in social organization. (Edited by H. R. Topoff.). W. H. Freeman & Co., San Francisco.
Smallwood, J. 1982. Nest relocations in ants. Insectes Sociaux 29: 138-147.
Smith, M. R. 1962. A remarkable new Stenamma from Costa Rica, with pertinent facts on other Mexican and Central American species (Hymenoptera: Formicidae). J. N. Y. Entomol. Soc. 70: 33-38.
Swartz, M. B. 1998. Predation on an Atta cephalotes colony by an army ant, Nomamyrmex esenbeckii. Biotropica 30: 682-684.
Tho, Y. P. 1981. A Unique Defense Strategy in the Termite Prohamitermes mirabilis (Haviland) of Peninsular Malaysia. Biotropica 13:236-238.
Tho, Y. P., and U. Maschwitz. 1988. The use of prefabricated plugs for emergency entrance sealing: a unique defense strategy in termites. Naturwissenschaften 75:527-528.
Watkins, J. F., II. 1964. Laboratory experiments on the trail following of army ants of the genus Neivamyrmex (Formicidae: Dorylinae). J. Kansas Entomol. Soc. 37: 22-28.
Wheeler, W. M. 1910. Ants: their structure, development and behavior. Columbia University Press, New York.
Page author:
John T. Longino, The Evergreen State College, Olympia WA 98505 USA.longinoj@evergreen.edu