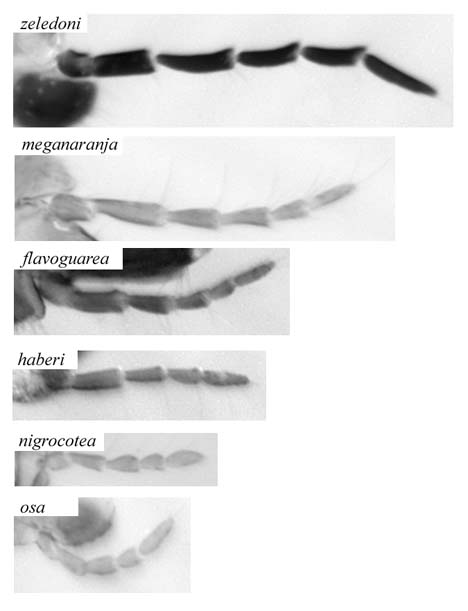
Figure 1. Examples of Myrmelachista worker maxillary palpi, showing reduction from 6 to 5 segments.
| Genus List | Key to Species | Species list |
Within the ants a number of lineages have developed exquisitely arboreal habits, nesting entirely within plant cavities and with specialized morphology and behavior for doing so. Species within these clades often show a range of specialization, being generalist inhabitants of dead stems, generalist inhabitants of live stems, or specialist inhabitants of live stems. The third group is often involved in obligate associations with particular lineages of plants and is particularly important in the study of mutualism (Davidson and McKey 1993).
Among these stem-nesting ants is the formicine genus Myrmelachista. This group of ants is confined to the Neotropics and is exclusively arboreal. They are inconspicuous and have been little studied, but there are hints from the literature that they are far richer and with more complex plant associations than previously suspected. Early literature contained scattered reports of South American Myrmelachista inhabiting ant-plants (reviewed in Bequaert 1922, Wheeler 1942). Myrmelachista nigella Roger and M. schumanni Emery were reported in internodes of Duroia hirsuta (Rubiaceae) (Ule 1907-1908, Schumann 1889). More recent reports have described "devil's gardens" in South America, in which large polygynous colonies of Myrmelachista occupy monospecific patches of understory Duroia and Tococa (Melastomataceae) (Morawetz et al. 1992, Svoma and Morawetz 1992, Renner and Ricklefs 1998, Frederickson et al. 2005). These patches stand out in the typically dense and diverse understory vegetation as peculiar zones of a single plant species with bare earth beneath them and a ring of bare soil around the perimeter. These patches are created and maintained by the ants, which attack and kill foreign vegetation by spraying it with formic acid, which acts as an herbicide (Morawetz et al. 1992, Renner and Ricklefs 1998, Frederickson et al. 2005).
The first indication of something interesting in Central American Myrmelachista was Stout's (1979) observation of a tight association between an unidentified Myrmelachista and an understory tree species in the genus Ocotea (Lauraceae). She examined 50 plants of Ocotea "pedalifolia" (probably a mix of O. atirrensis and O. dendrodaphne [Hammel 1986]) at the La Selva Biological Station, and found the stems of 49 of them inhabited by Myrmelachista. Since then little has been written about the association, although floristic works on the Lauraceae recognize that various species are routinely occupied by ants (Hammel 1986, Burger and van der Werff 1990). Ibarra-Manríquez and Dirzo (1990) reported the same phenomenon at the Los Tuxtlas Biological Station in Veracruz, Mexico. Longino (2006) showed that these observations are just the beginning and that there is a largely overlooked community of Central American Myrmelachista and their associated plants.
Throughout Costa Rica the understory of mature wet forests have high densities of plants that are associated with multiple species of Myrmelachista. Cloud forests likewise have high densities of Myrmelachista living in live stems, but they are more abundant in the canopy than in the understory. Many of these species nest entirely inside of live stems and rarely venture out onto the surface, and the plants they inhabit show no external signs of specialization for ant occupation. There are no preformed domatia, no food bodies, and no extrafloral nectaries. The result is a lineage of ants that in spite of being species rich and very abundant is almost never collected. Many of the species reported here had never been collected prior to my work in Costa Rica, much less receiving any formal taxonomic treatment.
GENERIC PLACEMENT AND DIAGNOSIS
Bolton (2003) placed Myrmelachista in the tribe Plagiolepidini, in the lasiine tribe group. The diagnosis for the tribe group included (1) widely separated metacoxae and (2) the petiolar foramen long, extending to or beyond the anteriormost points of the metacoxal cavities. The diagnosis for the Plagiolepidini included (1) the petiolar node inclined anteriorly, or with a long posterior peduncle, or both; and (2) the base of abdominal segment III with complete tergosternal fusion on each side of the helcium, with the free tergite and sternite commencing some distance up the sclerite, well away from the helcium. Ants of the genus Myrmelachista can be distinguished from other plagiolepidines by having a 9 or 10-segmented antenna with a 3 or 4-segmented club. Other genera have the antenna with more than ten segments and/or the funiculus filiform or gradually thickening toward the apex (Bolton 2003). Other plagiolepidine genera with 9 or 10-segmented worker antenna are Aphomomyrmex, Brachymyrmex, and Petalomyrmex. Although Bolton (2003) listed 6,4 as the palpal formula for Myrmelachista, some of the species reported here have 5-segmented maxillary palpus.
The taxonomic history of the genus Myrmelachista is covered amply by Bolton (2003). Briefly, Roger (1863) described two genera, Myrmelachista and Decamera, that differed in number of antennal segments, nine in the former and ten in the latter. Decamera was a junior homonym and was changed to Hincksidris by Donisthorpe (1944). Hincksidris (earlier as Decamera) was placed as a subgenus of Myrmelachista by various authors and ultimately synonymized under Myrmelachista (Snelling and Hunt 1976).
There are 79 available names in the genus, of which only six are currently junior synonyms (Bolton 1995). Although it was recognized that the number of antennal segments could not be relied upon to reveal monophyletic taxa and that the division of Myrmelachista into two groups was artificial (Brown 1973, Snelling and Hunt 1976), number of antennal segments is stable within species and there is geographic patterning with respect to the abundance of one form versus the other. Among the 73 valid species-group taxa, 27 have workers with 9-segmented antenna and 46 have workers with 10-segmented antenna. The 9-segmented forms are mostly concentrated in Central America and the Caribbean, with only two known from South America. The 10-segmented forms are mostly from South America, with only 3 from Central America and Mexico.
MEASUREMENTS AND INDICES
On these web pages, the following terminology and abbreviations are used:
HL: head length in full face view; perpendicular distance from line tangent to rearmost point of vertex margin to line tangent to anteriormost projection of clypeus (either laterally near mandibular insertion or median lobe, depending on which extends further), not including anteromedian denticle if present.
HW: head width; in full face view, maximum width of head capsule above eyes (not including eyes if they project beyond border of head capsule).
SL: scape length; length of scape shaft from apex to basal flange, not including basal condyle and neck.
EL: eye length, measured along maximum diameter.
OW: width of median ocellus.
OD: distance between inner margins of lateral ocelli.
CI: cephalic index; 100*HW/HL.
OI: ocular index; 100*EL/HW.
OcI: ocellar index, 100*OW/HL.
SPECIES DIAGNOSES
Species accounts are provided with a diagnosis. There are not separate diagnoses for workers, queens, and males; the diagnosis is the combination of worker, queen, and male characters that together most easily distinguish the species.
WORKER CHARACTERS
Workers show a great deal of plasticity and are not easily separated to species. There is inter- and intracolony size variation, and larger workers tend to exhibit more differences among species than small workers. The only sharply defined character with two discrete states is the number of antennal segments: 9 or 10. All other characters are continuous, with each species showing a mean and a variance, overlapping with similar species, but not encompassing the entire range of variation. The maxillary palpus may be 5-segmented, 6-segmented, or both (Fig. 1). In some cases the terminal palpomere is elongate and there is a slight constriction in the middle, suggesting partial fusion of the terminal two palpomeres. In some cases within a species (e.g., plebecula) colonies with small workers have 5 segments, colonies with larger workers have 6. There are occasional individual specimens with one 5-segmented palpus and one 6-segmented palpus.

Figure 1. Examples of Myrmelachista worker maxillary palpi, showing reduction from 6 to 5 segments.
Mandible sculpture varies from smooth and shining to coarsely punctatorugose.
Pilosity patterns are quite variable but show trends within species. In full face view, pilosity on the side and rear margins may be sparse or abundant, very short or longer, about the length of the first funicular segment, fully appressed to suberect. Among the Costa Rican species pilosity on the scapes is always abundant and suberect to subdecumbent, and I have not found it useful for identification. The pilosity on the outer margin of the hind tibia is always present as a covering of short setae of relatively uniform length, with variable presence of longer setae projecting above them. The setae may look rather uniform and short, with length about 1/4 width of tibia, or they may be longer and somewhat more irregular, the longer setae being 1/2 to 3/4 tibia width. The setae may be fully appressed to suberect.
Color is a useful character (Fig. 2). A set of seven species that have become obligate inhabitants of live stems of particular species of Lauraceae and Meliaceae have a uniform light yellow orange color, with variable development of infuscated bands on the gastral terga. Six species that are more generalized in their nesting habits and more often forage on the surface have largely brown coloration, with variable development of mottling of the mesosoma. Problematic cases are when workers are bicolored, with light colored head and mesosoma and dark colored gaster. Myrmelachista plebecula, one of the surface foraging species, can approach the coloration of the some of the yellow species.

Figure 2. Myrmelachista workers. A. zeledoni. B. joycei. C. flavocotea. D. plebecula. Scale applies to entire figure.
I have rarely found useful characters in the shape of the mesosoma or petiole, although the species with 10-segmented antenna have a stronger hourglass shape, with strong constriction at the metanotal groove (Fig. 2).
QUEEN CHARACTERS
Queens are often more powerful differentiators of species than workers. The meristic, sculptural, and pilosity characters discussed for workers also apply to queens. Color is a valuable character, often sharply separating species when worker color does not. For example, the species that are specialist inhabitants of Ocotea and Guarea all have relatively uniform yellow workers, yet the queens differ dramatically in color, being either solid orange, solid black, or bicolored. Details of queen head size, head shape, and ocellus size are important for differentiating species.
MALE CHARACTERS
Males vary somewhat in the relative size of the ocelli, but the primary differences are in the genitalia (Fig. 3). Terminology follows Snodgrass (1941). The pygostyles are small setose papillae projecting from beneath the posterior margin of the 8th abdominal tergite. These may be present and easily visible or variously reduced to near invisibility or absence. The basiparamere is always present and large. It usually has a pronounced dorsal lobe that is similar to but shorter than the paramere. It varies from broadly triangular and blunt to long, thin, and needle-like. In the smallest males the lobe may be very short or absent. The paramere is articulated to the basiparamere and is always present and elongate, sometimes broad at the base and tapering to a blunt point, or broad and parallel at the base and then tapering to a blunt point (looking like a seal head in profile), or long and thin with somewhat parallel sides, tapering to a point at the end. Internal to the paramere is the volsella, comprising two parts, the cuspis and the digitus. The cuspis is external to the digitus and closely applied to the inner wall of the paramere. The relationship of cuspis to digitus is somewhat like human thumb to clasped fingers. The cuspis is always shorter than the digitus and may be widely separated from it or with the apex converging on the dorsal margin of the digitus. The cuspis may be large, heavily sclerotized, and paddle shaped. From this state it shows reduction to a thin rectangular strip, to a small pointed needle-like structure, to a tiny triangular remnant fused to the inner margin of the paramere, and finally to completely absent. The digitus varies a great deal in shape, from a short, sharply downturned triangle, to a long tapering blade, to a scimitar-shaped blade that becomes broader toward the apex before narrowing, to a blunt paddle-shaped structure. Internal to the volsella is the aedeagus, comprising two penial valves. The penial valve has an anterodorsal apodeme, a broad and elongate blade, and a posterior upturned tooth. The apodeme may meet the dorsal margin of the blade at nearly a right angle, resulting in a strongly upturned aedeagus relative to the main axis of the abdomen, or the apodeme may gently curve into the dorsal margin and project back at an obtuse angle, such that the aedeagus is not as strongly upturned in side view.
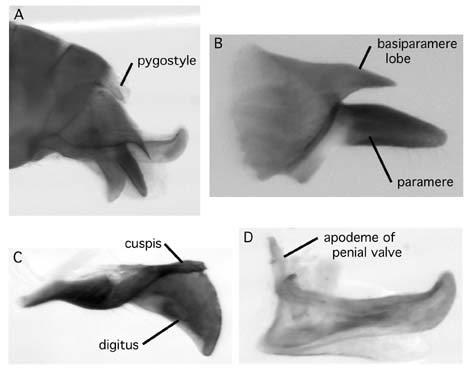
Figure 3. Male genitalia of Myrmelachista. A. Lateral view of male abdomen, showing undissected genitalia. B-D are dissected structure in same orientation and in order from outer to inner. B. Basiparamere and paramere. C. Volsella, a three-dimensional structure with cuspis external to digitus. D. Penial valve. A, B, D: joycei; C: nigrocotea.
Within species there is variation in degree of sclerotization of males, accompanied by variation in the size and robustness of genitalic elements. Less heavily sclerotized males also seem to have narrower and shorter basiparamere lobes, parameres, and volsellae. The variation is primarily among colonies and variation within colonies is low. The cause of high inter-colony variation is unknown. It could be due to ontogenetic changes in synchronous batches of males, intraspecific variation that is either genotypic or phenotypic, or evidence of cryptic species.
Literature Cited
Bequaert, J. (1922). Ants in their diverse relations to the plant world. Bulletin of the American Museum of Natural History 45, 333–584.
Bolton, B. (1995). A New General Catalogue of the Ants of the World. Cambridge, Mass., Harvard University Press.
Bolton, B. (2003). Synopsis and classification of Formicidae. Memoirs of the American Entomological Institute 71, 1–370.
Brown, W. L., Jr. (1973). A comparison of the Hylean and Congo-West African rain forest ant faunas. Tropical forest ecosystems in Africa and South America: a comparative review. Meggers, B. J., Ayensu, E. S. and Duckworth, W. D. Washington, D.C., Smithsonian Institution Press, 161–185.
Burger, W. and van der Werff, H. (1990). Flora Costaricensis, Family #80 Lauraceae. Fieldiana, Botany 23, 1–129.
Davidson, D. E. and McKey, D. (1993). The evolutionary ecology of symbiotic ant-plant relationships. Journal of Hymenoptera Research 2, 13–83.
Donisthorpe, H. (1944). Notes arising out of Mr. W. D. Hincks's review of Mr. Donisthorpe's "A list of the type-species of the genera and subgenera of the Formicidae". Entomologist's Monthly Magazine 80, 59.
Frederickson, M. E., Greene, M. J. and Gordon, D. M. (2005). 'Devil's gardens' bedevilled by ants. Nature 437, 495–496.
Hammel, B. E. (1986). The vascular flora of La Selva Biological Station, Costa Rica - Lauraceae. Selbyana 9, 218–233.
Ibarra-Manríquez, G. and Dirzo, R. (1990). Arboreal myrmecophilous plants from Los Tuxtlas Biological Station, Veracruz. Revista de Biologia Tropical 38, 79–82.
Longino, J. T. (2006). A Taxonomic review of the genus Myrmelachista (Hymenoptera: Formicidae) in Costa Rica. Zootaxa 1141:1-54.
Morawetz, W., Henzl, M. and Wallnöfer, B. (1992). Tree killing by herbicide producing ants for the establishment of pure Tococa occidentalis populations in the Peruvian Amazon. Biodiversity and Conservation 1, 19–33.
Renner, S. S. and Ricklefs, R. E. (1998). Herbicidal activity of domatia-inhabiting ants in patches of Tococa guianensis and Clidemia heterophylla. Biotropica 30, 324–327.
Roger, J. (1863). Die neu aufgeführten Gattungen und Arten meines Formiciden-Verzeichnisses nebst Ergänzung einiger früher gegebenen Beschreibungen. Berliner Entomologische Zeitschrift 7, 131–214.
Schumann, K. (1889). Ueber Ameisenpflanzen. Sammlung gemeinverständlicher wissenschaftlicher Vorträge (Hamburg) 4 , 421–459.
Snelling, R. R. and Hunt, J. H. (1976 [1975]). The ants of Chile (Hymenoptera: Formicidae). Revista Chilena de Entomología 9, 63–129.
Snodgrass, R. E. (1941). The male genitalia of Hymenoptera. Smithsonian Miscellaneous Collections 99, 1–86.
Stout, J. (1979). An association of an ant, a mealy bug, and an understory tree from a Costa rican rain forest. Biotropica 11, 309–311.
Svoma, E. and Morawetz, W. (1992). Drüsenhaare, emergenzen und blattdomatien bei der ameisenpflanze Tococa occidentalis (Melastomataceae). Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 114, 185–200.
Ule, E. (1907-1908). Die Pflanzenformationen des Amazonas-Gebietes. Pflanzengeographische Ergebnisse meiner in den Jahren 1900-1903 unternommenen Reisen. Botanische Jahrbucher für Systematik, Pflanzengeschichte und Pflanzengeographien 40, (1907) 114–172, (1908) 398–443, 8 plates.
Wheeler, W. M. (1942). Studies of Neotropical ant-plants and their ants. Bulletin of the Museum of Comparative Zoology 90, 1–262.
Page author: John T. Longino longinoj@evergreen.edu
Date of this version: 20 March 2006.