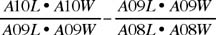
| Genus List | Key to Species | Species list |
Much of the material on these Crematogaster pages is copied directly from Longino, J. T. 2003. The Crematogaster (Hymenoptera, Formicidae, Myrmicinae) of Costa Rica. Zootaxa 151:1-150.
Contents:
Overview of genus
Natural History Overview
Morphology
Measurement Definitions
Diversity and Relative Abundance
Acknowledgments
Literature Cited
Specimens examined list for Longino (2003, The Crematogaster of Costa Rica, Zootaxa).
Notes on other Neotropical Crematogaster species (click here).
The genus Crematogaster is a widespread and distinctive lineage of myrmicine ants (Wheeler 1910, Forel 1928, Buren 1959, 1968, Hoelldobler and Wilson 1990). Most species are tropical, where they form dominant elements of the arboreal fauna. Some species groups have crossed the frost line, radiating in temperate zones, where they more often nest in the ground and beneath stones (Wheeler 1906).
The genus as a whole is clearly monophyletic, with a unique and apomorphic arrangement of postpetiole and gaster. The gaster has a tear drop shape, pointed posteriorly, and the postpetiole attaches to the dorsal surface of the fourth abdominal tergite. Thus the gaster is suspended beneath the postpetiole rather than being clearly posterior to it. The petiole has no dorsal node, and when the gaster is elevated the petiole fits flush against the propodeum. This combination of characters is probably related to defensive or offensive behavior in which workers wave their gasters in the air, exuding a droplet of venom onto the spatulate sting (Buren 1959). Not all species exhibit this behavior (D. Davidson, pers. comm.) but most do. Workers are monomorphic or exhibit continuous size polymorphism, but there is no distinct major worker caste. The antennae are 11-segmented with the exception of one lineage of Asian and African species, in which there are 10 segments. The antennae have a terminal club of 2-4 segments. The propodeum usually has a pair of dorsal spines (lack of spines is rare among New World species), but otherwise the workers lack armor.
At the species level the genus can appear somewhat monotonous because there is little variation in major shape characters. Nevertheless species diversity is high, both within local communities and globally. Species differ in details of petiole and postpetiole shape, overall pilosity characteristics, and surface sculpture. Because the genus is a common and conspicuous element of most faunas, especially in the tropics, and varies both within and among regions, 889 available names have been generated (Bolton 1995). Nearly all of these have accumulated as unconnected species descriptions and there are very few synthetic works (Buren's 1968 review of North American Crematogaster s.s. a notable exception). Attempts have been made to recognize subgenera, but they are not well supported nor well defined. The high species diversity and lack of higher level taxonomic structure in the genus has made it one of the intractable messes in the world of ant taxonomy, on par with Solenopsis and Pheidole. It is a group generally avoided by students of systematics seeking manageable projects.
Hillis (1988) recognized three phases in the history of taxonomic understanding of the Rana pipiens complex: (1) a typological phase when valid names increased; (2) a "biological species" phase marked by extensive synonymy under widespread polytypic species, marked by a decrease in the number of valid names; and (3) a "phylogenetic species" phase during which the polytypic species resolve into complex mosaics of discrete species, and the number of valid names increases again. Although I am not a proponent of the phylogenetic species concept, the empirical progression in number of valid names does reflect progress. Following the first wave of typological description, a reevaluation is justified in which many names are synonymized under large polytypic species. An available name is considered a synonym until proven distinct, rather than the reverse. Thus a synonymization is not a strong statement that two forms are part of the same biological species, which is an unprovable null hypothesis, but instead is an inability to reject the null hypothesis of conspecificity. Further investigation may discover character or geographic evidence of species differences and require a revalidation of the synonym. Large polytypic species with many synonyms are expected to resolve into multiple species when more data are at hand.
I took a regional (rather than global) approach in my review of the Costa Rican species (Longino 2003) that resulted in a hybrid of the second and third phases. Some species found in Costa Rica were recognized as members of widespread polytypic species, and extensive synonymy was proposed based on little more than an examination of the types. Within Costa Rica, more extensive collections allowed a more thorough examination of character variation, and a number of discrete sympatric or parapatric forms were revealed. The new species were rare or geographically restricted forms that has been overlooked in the early typological description phase. Similar faunistic studies in other regions are expected to yield similar results by finding a set of common widespread species that already have names and a set of rare or geographically localized species that are new.
Crematogaster are often common ants, and they play a major ecological role in Neotropical forests. Colonies may be large, blanketing forest canopies, or small, contained within a single dead twig. Large colonies are usually polydomous, with multiple nests. Most species nest in dead wood, from narrow gauge hollow stems to large dead branches or trunks. One species, C. stollii, nests in live stems. Although major Crematogaster lineages in the Asian and African tropics are specialized plant ants, and at least one or two species are plant ants in the Amazonian region, none are known to be specialized plant ants in Costa Rica. Crematogaster bryophilia often nests under epiphyte mats. Although many species can make carton from masticated plant fibers, most use relatively small amounts to form partitions inside the nest or to restrict the opening of a nest in dead wood. Several Costa Rican species use carton more extensively. Crematogaster stollii makes carton galleries on tree trunks and branches, connecting their nests in the live branch tips. These carton galleries are indistinguishable from those of Azteca forelii, an ant species with similar nesting behavior, and both are very similar to the galleries of the arboreal termites that are so common in lowland forests. Crematogaster montezumia and C. arcuata make external carton nests that encircle small stems. These nests are plain carton, and lack epiphytes. In contrast, two Costa Rican species make carton nests that sprout epiphytes, forming ant gardens. Crematogaster longispina makes loose ant gardens on tree trunks (Kleinfeldt 1978), and C. jardinero lives in the high canopy, forming "archipelago" clusters of discrete ant gardens.
Although most species are arboreal, a few nest in the leaf litter. Species that nest in the leaf litter are usually yellow, nocturnal, and rarely encountered. One leaf litter species, C. sotobosque, is brown, forages diurnally on low vegetation, and is moderately abundant in lowland wet forest.
Most species are monogynous; a few are polygynous. Ergatogynes or intercastes have been reported for C. minutissima (Holliday 1903), C. minutissima smithi, and a species tentatively identified as C. curvispinosa (Heinze et al. 1998, Heinze et al. 1999). The Heinze et al. studies of smithi revealed that these intercastes, morphologically intermediate between workers and queens, function mainly to provide trophic eggs for the colony. They never perform foraging, maintenance, or defensive duties. They mainly lay eggs, most of which are eaten by larvae. The ergatogynes lack a spermatheca and cannot be inseminated, but their eggs are viable and produce males if left to develop. Among the Costa Rican fauna, ergatogynes are known to occur in C. bryophilia, C. curvispinosa, and C. nigropilosa. Nests are often found with only ergatogynes, workers, and brood. It is unknown whether these are colony fragments, with queenright nests elsewhere, or whole colonies founded by ergatogynes.
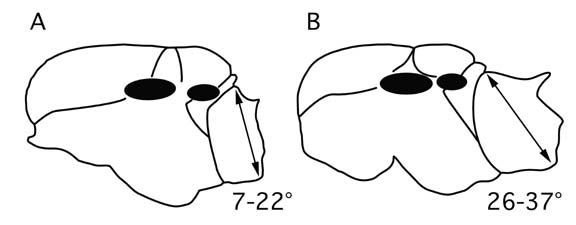
Two categories of queens occur among the Costa Rica fauna. In one group the propodeum is tall and narrow and drops very steeply from the scutellum (Fig. 1A), and sculpture and pilosity characters are similar to workers. I refer to these as "normal" queens. These species appear to have typical colony founding behavior, with standard nuptial flights and claustral colony founding by individual queens, and they are the most abundant species in communities. In another group the propodeum has a shallower slope and extends well beyond the scutellum (Fig. 1B), and sculpture and pilosity characters often differ greatly from workers. In particular, queens are often highly polished and shiny. Queens in this group also show varying degrees of development of falcate mandibles. Costa Rican species having these distinctive queens are acuta, arcuata, distans, evallans, jardinero, montezumia, and raptor. I refer to these as the "acuta-group." These are all very low density species, and very little is known of their colony founding behavior. The morphology is similar to other ant lineages that are known to be temporary social parasites (Forel 1928, Hlldobler and Wilson 1990). Queens of temporary social parasites insinuate themselves into nests of other species, killing or incapacitating the host queen, and use the heterospecific worker force to establish their own colony. Two anecdotal observations are consistent with temporary social parasitism as a colony founding mechanism in the acuta-group. I observed a mixed nest in which a queen of C. montezumia occurred in a small nest with workers of C. curvispinosa, and Adrienne Nicotra, a student working at La Selva Biological Station, observed a queen of C. raptor in a small queenright nest of C. carinata. These are the only such observations so far, and the colony founding behavior of acuta-group species is in need of investigation.
Crematogaster appear to be very generalized and omnivorous foragers. Individual scouts search for resources and recruit nestmates when resources are encountered. They rapidly recruit to baits of sugar or protein (e.g., tuna, dead insects). Although rarely predators of active prey, I have often seen them attacking pupae or otherwise immobilized live prey. They readily tend Homoptera, and species vary in the degree of reliance on Homoptera. Crematogaster stollii appears to rely entirely on Homoptera and perhaps cryptic plant resources. Workers are found only inside of live stems or under their carton galleries and they never forage on the surface. Their chambers in live stems are packed with Coccoidea that are feeding from the inside of the stems. This phenomenon, in which ants live and feed entirely within live plant stems, with no external patrolling by the ants and no obvious myrmecophytic adaptations on the plant's part, has evolved convergently in several ant lineages, including species of Azteca and Myrmelachista.
In Costa Rica, Crematogaster are abundant in all lowland habitats. In mangroves, C. crinosa is often a dominant species. In lowland dry or wet forest sites, a community of over 15 species may occur. These are concentrated in second growth vegetation, forest edges, and forest canopy. Relatively few species are found in wet forest understory and forest floor litter. At higher elevations the dominant Crematogaster drop out by about 500 to 1000m, depending on the openness of the habitat. A few species, such as C. bryophilia, C. moelleri, and C. sumichrasti, are montane forest specialists that are more common at mid elevations than at sea level.
Workers
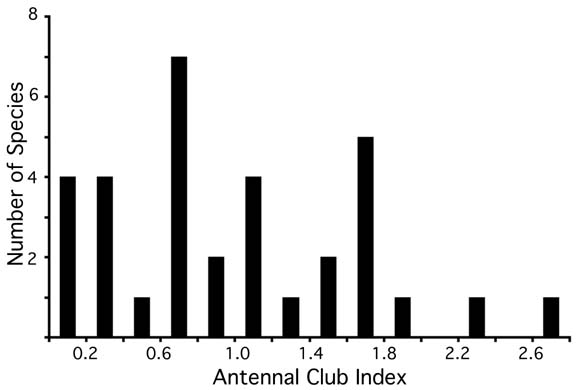
The terminal segments of the antenna are enlarged to form the antennal club. The club may be composed of the terminal two segments and sharply differentiated from segments eight and nine, or it may be composed of the terminal three or four segments, which gradually decrease in size. Although the number of segments in the club has been used as a subgeneric character (Santschi 1918), I find continuous interspecific variation (Figure 2) that does not correlate with other characters, and I do not use the character in the key.
The ventral margins of the petiole and postpetiole exhibit varying degrees of development of an anterior tooth. These anteroventral teeth are independent of each other, appearing on the petiole alone, on the postpetiole alone, on both, or on neither. They appear to be evolutionarily labile because they are not highly correlated with other characters and do not unite clusters of related species the way petiole shape seems to. Nevertheless, they are often reliable species-level characters: they correlate well with particular suites of characters that are hypothesized to be diagnostic for particular species. A clear view of the anteroventral margin of the petiole and postpetiole is often essential for species identification.
The postpetiole is comprised of the thickened rim of the anterior helcium, the narrow "neck" of the helcium, and the broadened and elevated node. The neck of the helcium may be short and cylindrical, forming a relatively sharp juncture with the node, like a stem on an apple. Alternatively, the neck may be relatively elongate and gradually tapering such that the juncture with the node is not sharply defined. In this case the dorsal view of the petiole is somewhat hour-glass or flask shaped. In the latter case, the node is globular, about as wide as long, and with no trace of a median longitudinal sulcus. In the former case the node is less globular, relatively more dorsally compressed, subquadrate in dorsal view, and usually distinctly wider than long. When the node is broad and subquadrate, it may exhibit some degree of development of a median longitudinal impression or sulcus. At extremes, the sulcus is very well defined, dividing the postpetiolar node into two distinct lobes, with a strong posterior emargination and a fully impressed sulcus.
Pilosity characters, like petiolar characters, are very useful and seem to unite clusters of related species. Pilosity on the face usually falls into one of three categories: (1) abundant long erect flexuous setae; (2) abundant short stiff setae, forming a stubble of straight or curved setae; or (3) largely or entirely bare, with a vestiture of sparse, very short, completely appressed hairs (a very dilute and appressed pubescence) and a small number of longer erect setae (Fig. 3). Pilosity on the meso and metasomal dorsum varies in density and may be composed of long flexuous or short stiff setae. Tibial and scape pilosity may be appressed or suberect and varies in length. When tibial pilosity is erect, it is usually subequal in length to the maximum width of the tibia, but in a few species there are 1-3 specialized longer setae that are about twice as long at the others.

Queens
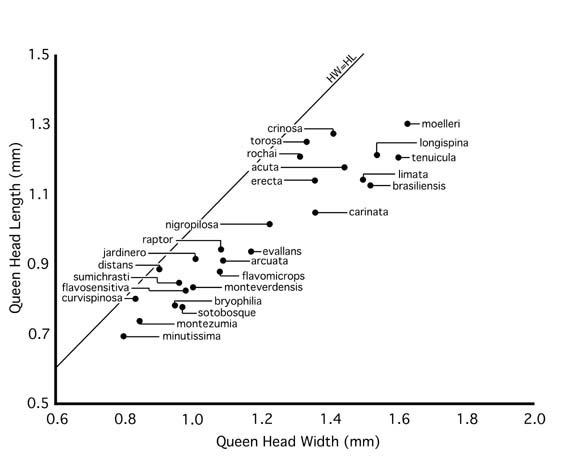
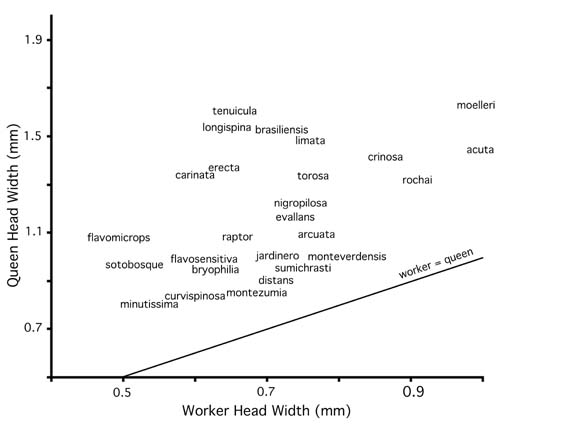
Queens are known for 27 of the 33 species that occur in or near Costa Rica. Normal queens are similar to workers in general shape, sculpture, and pilosity characters, differing in typical caste-specific traits: larger size, ocelli, enlarged mesosoma with additional sclerites, wings or wing scars, and enlarged gaster. As discussed in the Natural History Overview, acuta-group queens are more strongly differentiated from workers, often with highly polished integument and/or distinctive pilosity traits. There is variation in queen size (Fig. 4) and in the relationship of queen size to worker size (Fig. 5). Queens are given very cursory treatment in this work. Size relationships are depicted in Figures 4 and 5, and brief descriptions are provided in the species accounts.
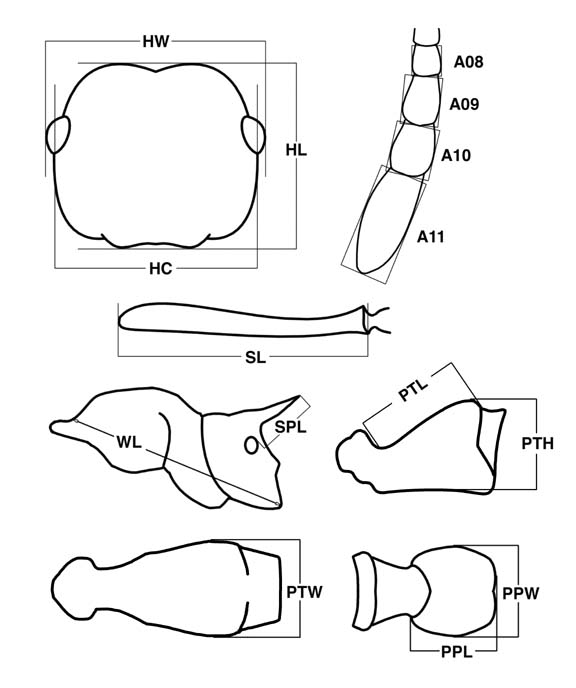
HL: head length; perpendicular distance from line tangent to rearmost points of vertex margin to line tangent to anteriormost projections of clypeus, in full face view.
HW: head width; maximum width of head in face view, including eyes if they project beyond the sides of the head.
HC: head capsule width; maximum width of head in full-face view, excluding eyes; may be anterior or posterior to eyes.
SL: scape length; length of scape shaft from apex to basal flange, not including basal condyle and neck.
EL: eye length, measured along maximum diameter.
A11L, A10L, A09L, A08L: length of 11th to 8th antennal segment.
A11W, A10W, A09W, A08W: width of 11th to 8th antennal segment.
WL: Weber's length; viewing mesosoma in lateral profile, distance from approximate inflection point, where downward sloping pronotum curves into anteriorly projecting neck, to posteroventral propodeal lobes.
SPL: propodeal spine length; measured from tip of propodeal spine to closest point on outer rim of propodeal spiracle.
PTH: petiole height; viewed in lateral profile, perpendicular distance from ventral margin to highest point of posterolateral tubercles; if ventral margin is concave upward, measured from line tangent to uppermost portion of curve and oriented as close as possible to long axis of petiole.
PTL: petiole length; viewed in lateral profile and measured in same plane as anterodorsal face, distance from inflection point marking juncture of posterolateral lobes and cylindrical posterior portion of segment to anterior inflection point where petiole curves up to condyle or, if inflection point not visible, where petiole is obscured by posteroventral lobes of propodeum.
PTW: petiole width; maximum width of petiole in dorsal view.
PPL: postpetiole length; viewing at an angle that maximizes length (approximately parallel to fourth abdominal tergite), perpendicular distance from line tangent to anterior inflection point (narrowest point when postpetiole is hourglass-shaped where it joins the helcium, point immediately anterior to node when helcium is sharply differentiated from node as a distinct cylindrical stem) to line tangent to posteriormost lobes if bilobed, to posteriormost point if globular.
PPW: postpetiole width; maximum width of postpetiole, in same view as and perpendicular to postpetiole length.
CI: cephalic index; 100*HW/HL.
OI: ocular index; 100*EL/HL.
SI: scape index; 100*SL/HL.
SPI: propodeal spine index; 100*SPI/WL.
PTHI: petiole height index; 100*PTH/PTL.
PTWI: petiole width index; 100*PTW/PTL.
PPI: postpetiole width index; 100*PPW/PPL.
ACI: antennal club index;
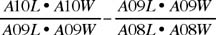
This index attempts to quantify the degree of distinctness of the club. The product of segment length and width is a proxy of overall segment size, and the index measures relative change in size from segment 10 to 9 compared to relative change from 9 to 8. A sharply differentiated two-segmented club will have segment 10 much larger than 9, and segment 9 about the same size as 8, and the index will be greater than zero. A club in which segments 10 to 8 gradually decrease in size will have similar first and second terms in the formula, and the index value will be near zero. A distinctly 3-segmented club will have a value less than zero.
For each species account three workers were measured. An attempt was made to select a small, medium, and large worker, based on the entire geographic range of the species, and each from a different nest series. Dimensions of antennal segments were measured for only one specimen; other measurements were made for all three workers. Each species account has a measurement list in which each measurement acronym is followed by the three values for the three workers, in order, such that measurements for individual workers can be reconstructed. Antennal segment dimensions, which have only one value, are for the first worker in the sequence. Measurements are also provided for holotypes. One queen for each species, when available, was measured for head length and head width.
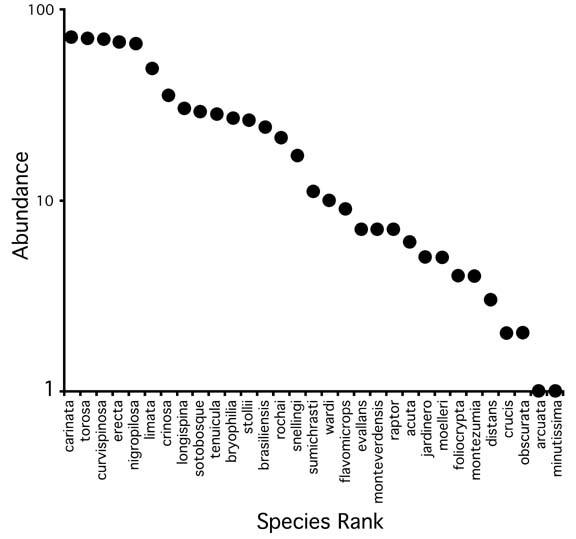
The known Costa Rican fauna is comprised of 31 species. The relative abundance relationships show a typical dominance diversity curve (Fig. 7). The data on which the curve is based are the result of subjective collecting methods by multiple collectors, rather than a more quantitative sampling program, but can nevertheless provide a rough estimate of the relative abundance distribution in the country, and can inform researchers of the likelihood of encountering particular species. A more quantitative assessment of a local Crematogaster community can be made using the results of the Project ALAS sampling at La Selva Biological Station in the Atlantic lowlands of Costa Rica (Table 1). The dominance diversity curve for this subset of 16 species is similar to the curve for the country as a whole. Table 1 also reveals the microhabitat preferences of the common species, with some species more frequently captured in fogging and Malaise samples, others more frequently found using methods that sample the forest floor.
The following curators were very helpful in the loan of specimens and during museum visits: Roy Snelling (LACM), C. Besuchet, D. Burckhardt, and I. Lbl (MHNG), V. Raineri (MCSN), S. Cover (MCZC), D. Smith (USNM), and P. Ward (U.C. Davis). The work benefited greatly from the abundant collections and generous contributions of Bill Mackay and Phil Ward. Casey Richart assisted with Automontage image preparation, and Melissa Barrows assisted with specimen measurements. Phil Ward thoroughly edited much of the text that went into this site. This work has been supported by the National Geographic Society, and The National Science Foundation grants BSR-9025024, DEB-9401069, DEB-9706976, and DEB-0072702.
 Casey Richart |
 Melissa Barrows |
Specimens examined for Longino 2003, Crematogaster of Costa Rica paper (Excel file, tab-delimited text file). Specimen codes that have "JTL" followed by a number are for material that does not have unique codes on the specimens themselves, and the specimen code is only in the database. All other codes, including those with "JTLC" followed by a number, are unique physical specimen codes on the pin, with human-readable text and machine-readable symbol. Collection code is a relational database linking field that may or may not be printed on the physical label. Locality data are not always exact transcriptions of specimen labels.
Page author: John T. Longino longinoj@evergreen.edu
Date of this version: 4 March 2003.