| Genus List | Species List | Key to Species |
The genus Acropyga in Costa Rica
The ant genus Acropyga is a fascinating group of formicine ants, found throughout the world in warm temperate and tropical areas. It is the relationship that Acropyga have with mealybugs that arguably has elicited the most interest in the genus. All Acropyga are thought to be hypogaeic (living entirely underground), surviving primarily, it is believed, by "tending" mealybugs (Hemiptera: Pseudococcidae) on underground roots for their exudate (sometimes referred to as "honeydew") (Weber 1944; Williams 1998). This mutually beneficial relationship is called trophobiosis (HÜlldobler and Wilson 1990). Evidence suggests that all Acropyga species are obligate coccidophiles, that the ants are dependent on the mealybugs for survival. And though many ant species are known to harvest honeydew from hemipterans, Acropyga have taken the relationship further. Queens of eleven species have been observed emerging from their nests prior to their mating flight with a mealybug held in their mandibles (Bčnzli 1935; Wheeler 1935; Brown 1945; Eberhard 1978; Prins 1982; Buschinger et al. 1987; Williams 1998; Johnson et al. 2001); the mealybug that each queen carries presumably serves as a "seed individual" from which a new generation of mealybugs will be started in the newly founded ant colony (Weber 1944; Williams 1998). This behavior has been termed trophophoresy by LaPolla et al. (2002), and queens that exhibit this behavior are said to be trophophoretic.
The complexity of the relationship between Acropyga and the mealybugs is not well understood, but it appears to be analogous to what has evolved between attine ants and the fungal species that they "farm." In support of a close relationship between mealybug and ant, Flanders (1957) found that A. fuhrmanni stores its own eggs with those laid by the pseudococcids found in the nest. It has also been observed that A. fuhrmanni move mealybugs from underground chambers where roots are found to chambers without roots. Flanders (1957) speculated that this was done in order to regulate the amount of honeydew produced in a colony and to protect the roots from overuse by the mealybugs. LaPolla et al. (2002) observed that A. epedana also keeps mealybugs with brood. Furthermore even when a nest in captivity was starved, workers refused a variety of food items presented to them, suggestive that the ants are completely dependent on the mealybugs as a food source. Fossil evidence suggests that the trophobiotic behavior of Acropyga ants is an ancient one. Johnson et al. (2001) reported that Acropyga queens were discovered in Dominican amber, either holding a mealybug or with a mealybug nearby in the amber matrix. The amber was dated to the Miocene, so the intimate relationship of Acropyga and mealybugs is at least 15-20 million years old.
|
Ant species
|
Mealybug species
|
Trophophoresy
|
|
A. exsanguis
|
G. coffeae [1], N. caldasiae [1], Neochavesia sp.(?) [1], Pseudorhizoecus sp. [1], P. proximus [1], R. caladii [1], R. coffeae [1], R. falcifer [1]
|
X
|
|
A. fuhrmanni*
|
Neochavesia sp. (eversi?) [1]
|
X
|
|
A. goeldii
|
Capitisetella migrans [1], Dysmicoccus radicis [1], Neochavesia sp. [1], R. coffeae [1]
|
X
|
|
A. keira
|
unidentified mealybug
|
|
|
A. panamensis*
|
mealybug morpho-1 [2]
|
|
|
A. smithii
|
G. coffea [1], N. eversi [1]
|
|
Table 1. Costa Rican Acropyga species known to be associated with mealybugs. Records of ants with mealybug species are not unique to Costa Rica but are instead from across of species known range. If the species has also been observed to display trophophoretic behavior that box is marked with an "X". Acropyga identities that LaPolla has confirmed with mealybugs are marked with "*". Acropyga marked with a "#" are species for which voucher material is known to exist for ant and mealybug, thereby making published identification more certain. Otherwise the identity of the Acropyga must be considered questionable until actual vouchers of ant and mealybug are made.
Reference:
[1] Johnson et al. (2002)
[2] D.J. Williams, personal communication
Species-level taxonomy
LaPolla (2004) recently revised the genus on a worldwide basis revealing 37 species. Eighteen species are presently known from the New World, with 7 species known from Costa Rica and all but one (A. panamensis is in the panamensis species-group) of those belong to the decedens species-group. One of the most difficult aspects of identifying these ants is their small size and pale color. Oftentimes placing a dark background behind a specimen can aid in observing characters. To identify most Acropyga species at some point examination of the mandibles will be necessary so it is important to always make certain some specimens have their mandibles open.
Species differences are often very subtle. Members of the decedens-species can be very difficult to distinguish from each other and in some cases males are needed to identify to the species level (i.e. goeldii and palaga). Consult LaPolla (2004) for aid in identifying male specimens.
Using the key
The key is a standard dichotomous key. It is designed so that it can be printed out on 8.5 x 11 inch paper if desired. The numbering sequence is not continuous but instead jumps in increments of about 10. This allows new species to be added or other modifications to be made without renumbering the entire document. The key has internal hyperlinks so that clicking on a "go to" number will take you to that couplet, and clicking on the number of the couplet itself will take you back to the couplet from which it came. This makes it easy to move up and down in the key, trying different directions. Species are hyperlinked to the species accounts, so that when you arrive at a species in the key you can view the image set and information about range, morphology, and natural history. If the identification does not look right, you can return to the same spot in the key and try another avenue.
It is apparent that workers and queens of some species display a remarkable range of phenotypic variation in size and shape of various characteristics, both within populations and over large geographical areas. When examined in isolation this variation could be thought to indicate species level differences. This in part has led to the large number of synonyms found in the genus. Even antennal segment number is variable in Acropyga, both within, and between species (Table 2). A single specimen may even display differing antennal segment numbers from one antenna to the other. An interesting trend is that the highest observed antennal segment number in workers is always one less segment than the highest antennal segment number observed in males. Changes in antennal segment number occur at segments 3-5 (numbered with scape as 1st segment), and despite changes in segment number the relative length of the flagellum does not change proportionally to it. For example, in A. goeldii, reduction from 11 to 10 segments occurs by fusion of segments 3 and 4. Further reduction to 9 segments entails fusion of segments 3, 4, and 5. This pattern appears to hold for all Acropyga species where variation in antennal segment number has been observed.
| Taxon | ASC | MTN |
| decedens spp. group | | |
| smithii | 7-8 | 4 |
| fuhrmanni | 8 | 4 |
| goeldii complex | | |
| exsanguis | 8-9 | 3 |
| goeldii | 9-11 | 3-4 |
| keira | 9 | 3 |
| palaga | 9-10 | 3 |
| panamensis spp. group | | |
| panamensis | 8 | 4 |
Table 2. Antennal segment and mandibular teeth number observed in Costa Rican Acropyga species. Note that antennal segment numbers often vary not only among species but also within a species. Queen ASC and MTN are identical with that of the reported worker unless otherwise noted on species pages. ASC= antennal segment count; MTN= mandibular teeth number.
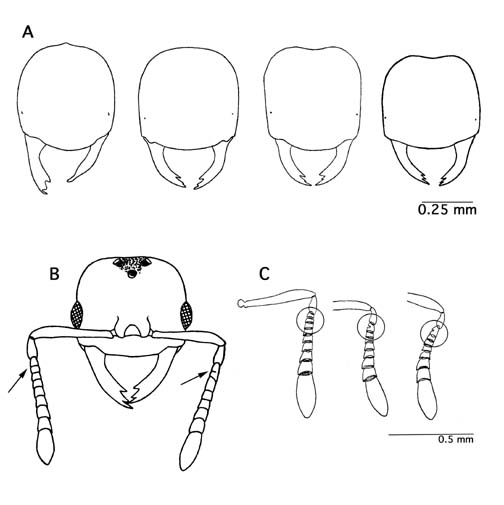
Figure 1. Variation observed within species. A) Bčnzli's (1935) drawings of head shape variation observed in A. exsanguis. B) An A. goeldii queen with differing number of antennal segments on each antennae (modified from Weber 1944). C) Antennae from 3 different A. goeldii workers depicting range of antennal segments posssible in the species (modified from Bčnzli 1935).
Nesting behavior
The small eyes, reduced antennae segmentation, lightly pigmented cuticle, and hairs covering the cuticle of Acropyga species are suggestive of a completely subterranean existence. Additionally, species display photophobic behavior (Weber 1944; LaPolla et al. 2002). Acropyga nests are found in leaf litter, under stones, in rotten wood (lying on or near the soil surface) and in the soil. Species in which nesting habits are known possess large nests, consisting of at least several thousand individuals, the structure of which is diffuse, with apparently no central nesting location (LaPolla et al. 2002). Tunnels and indistinct chambers stretch out over large areas through the nesting medium. Polygyny has been suggested for several species. The origins of polygyny in nests is uncertain, but two routes are suggested based on field observations. Bčnzli (1935) found both the occurrence of pleometrosis (founding of a colony by multiple queens) and the acquisition of young queens by established colonies in A. exsanguis.
Literature Cited
Brown, W.L., Jr. 1945. An unusual behavior pattern observed in a Szechuanese ant. Journal of the West China Border Research Society, 15: 185-186.
Bčnzli, G. H. 1935. Untersuchungen čber coccidophile Ameisen aus den Kaffeefeldern von Surinam. Mitt. Schweiz. Entomol. Ges. 16:453-593.
Buschinger, J. Heinze, J. & Jessen, K. 1987. First European record of a queen ant carrying a mealybug during her mating flight. Naturwissenschaften, 74: 139-140.
Eberhard, W.G. 1978. Mating swarms of a South American Acropygia [sic.] (Hymenoptera: Formicidae). Entomological News, 89(1 & 2): 14-16.
Flanders, S.E. 1957. The complete interdependence of an ant and a coccid. Ecology, 38, 535-536.
HÜlldobler B. & E.O. Wilson. 1990. The Ants. Belknap Press, Cambridge, Massachusetts, 732 pp.
Johnson, C., D. Agosti, J.H. Delabie, K. Dumpert, D.J. Williams, M. von Tschirnhaus, and U. Maschwitz. 2001. Acropyga and Azteca Ants with Scale Insects: 20 Million Years of Intimate Symbiosis. American Museum Noviates, 3335: 1-18.
LaPolla, J.S. 2004. Acropyga (Hymenoptera: Formicidae) of the World. Contributions to the American Entomological Institute 33:1-130.
LaPolla, J.S., S.P. Cover & U.G. Mueller. 2002. Natural history of the mealybug-tending ant Acropyga epedana, with descriptions of the male and queen castes. Transactions of the American Entomological Society, 128(3): 367-376.
Prins, A.J. 1982. Review of Anoplolepis with reference to male genitalia, and notes on Acropyga. Annals of the South African Museum, 89: 215-247.
Weber, N.A. 1944. The Neotropical coccid-tending ants of the genus Acropyga Roger. Annals of the Entomological Society of America, 37: 89-122.
Wheeler, W.M. 1935. Ants of the genus Acropyga Roger, with description of a new species. Journal of the New York Entomological Society, 43: 321-329.
Williams, D.J. 1998. Mealybugs of the genera Eumyrmococcus Silvestri and Xenococcus Silvestri associated with the ant genus Acropyga Roger and a review of the subfamily (Hemiptera, Coccoidea, Pseudoccidae). Bulletin of the British Museum (Natural History)(Entomology), 67: 1-64.
Page author:
John LaPolla, Department of Entomology, Rutgers University, New Brunswick, New Jersey 08901, USA. lapolla@eden.rutgers.edu
John T. Longino, The Evergreen State College, Olympia WA 98505
USA. longinoj@evergreen.edu
Date of this version: 14 July 2004.
Previous versions of this page:
 Go back to top
Go back to top
Go to Ants of Costa Rica Homepage
