
|
|
|
Introduction
Icaricia icarioides blackmorei (Barnes and McDunnough, 1919) is a butterfly that inhabits the Willamette Valley-Puget Trough-Georgia Basin ecoregion. It has an absolute dependence on its local larval host plant and, at least in lowland Puget Sound populations, it seems to depend on this host for adult resources as well.
Native grasslands, the lowland habitat of I. i. blackmorei, have declined in extent to about 2% of the area they occupied in 1850 throughout the Willamette Valley-Puget Trough-Georgia Basin ecoregion (Crawford and Hall 1997). This is primarily due to development for agriculture, urbanization, gravel mining, succession to forest, and non-native invasive species. Many species of prairie dependent vertebrate animals have declined dramatically and some are threatened with extinction or have already been extirpated from prairies (Leonard and Hallock 1997; Rogers et al. 1997; Rogers 2000; Ryan 1997). Plants of the prairie have declined as well. For example, the golden paintbrush (Castilleja levisecta) is federally listed as threatened under the Endangered Species Act. Several other plants are listed as “sensitive” under the Washington State Natural Heritage Program.
Work on insects, thus far mostly confined to butterflies, is beginning to show the same pattern of population decline and endangerment in prairie obligates. Butterflies in particular are declining, with four butterflies of the Puget Prairies listed as “endangered” or “species of concern” by the state of Washington and two species proposed for listing under the federal Endangered Species Act. Thirteen butterflies that use grasslands in the ecoregion are listed as endangered, threatened, candidate, or extirpated (or equivalents) by national or state/provincial governments. Recently a petition from several conservation groups was submitted to the U.S. Fish and Wildlife Service for emergency listing of three butterflies in the ecoregion (Vaughan and Black 2002a, 2002b). Butterflies are often considered to be both good indicator (Black et al. 2001; Pollard and Yates 1993; Samways 1994) and umbrella (Launer and Murphy 1994; New 1997) taxa. The imperiled nature of these taxa is particularly troubling and indicates, along with the dramatic reduction in habitat, that the prairie system is in crisis.
The higher level taxonomy of I.i. blackmorei places it in the tribe Polyommatini Swainson, 1827 within the subfamily Polyommatinae Swainson, 1827 of the Lycaenidae Leach, 1815. Icaricia icarioides has been classified in at least four different genera since it was named by Boisduval (1852). It started out in Lycaena, was transferred to Icaricia by Nabokov (Hodges et al. 1983), and a move to Aricia was attempted (Bálint and Johnson 1997). Gorbunov (2001) lumped several genera including Icaricia into supergenus Plebejus and this was accepted by Opler and Warren (2003). I reject this and accept Icaricia because the genera lumped under Plebejus appear to be natural groupings and each has distinctive shared apomorphies (Guppy and Shepard 2001). Emmel (1998) and Guppy and Shepard (2001) conservatively kept icarioides in Icaricia and this is the taxonomy accepted here. The taxonomy of this genus is still very much in flux and, in Gorbunov’s treatment Icaricia would be demoted to subgenus status.
Description
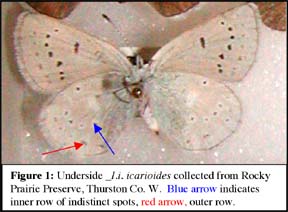
Icaricia icarioides blackmorei is a small butterfly (wingspread 21-33mm). This species has two rows of small white spots on the underside of the hind wing either with indistinct edged black spots or with black spots absent or nearly absent. The white spots are always present but they are highly variable. In many specimens they are just barely visible white spots slightly contrasting with the light grey ground color (Figure 1). The outer wing margin is fringed with fine white hairlike scales. The antennae are ringed in alternating bands of black and white throughout their length in both sexes.
The adult male has bright, light blue upper wing surfaces with a border of brown inside a faint white fringe along the wing margin (Figure 2). The ground color of the ventral surface of wings is light gray and overlaid with white and black scales, giving males a much lighter appearance overall on both dorsal and ventral surfaces.
Adult females have upper side of wings brown often with a blue sheen that is usually restricted to the basal area of the wing (Figure 3). The ground color of the wing underside is very light brown and this is overlaid with mostly white scales (Figure 2) with black scales restricted to the center of areas heavily scaled with white scales.

Immature
Larvae, like all larvae in this subfamily, are slug or chiton like in appearance. The head of the larvae is not visible from above. The early instar larvae are generally pale maroon to faintly green in color. Post diapause larvae become more green in later instars. Both pre and post diapause larvae seem to fairly closely match the color of the lupine on which they are feeding. Sometimes larvae can be found by observing ants tending the inconspicuous larvae. Pupae are green on the head and thorax, brownish red with green spots on the abdomen.
Diagnosis
This species is separated in the field from the other blue butterflies in the lowland Puget Trough and Georgia Straights area by two rows of small white spots on the underside of the hind wing either with indistinct edged black spots or with black spots absent (white spots immaculate) or nearly absent. The other Polyommatinae in this region can be separated by the arrangement or shape of spots on the underside of the hind wing and other wing characteristics. Everes amyntula (Boisduval, 1852) has fine tails on the edge of the wing extending from the first anterior cubitus branch vein. Celastrina echo (W.H. Edwards, 1864) has marginal spots and faint smudge like submarginal chevrons—both without distinct white halos. Glaucopsyche lygdamus (Doubleday, 1841) has a single uneven postmedian row of distinct black spots ringed with white (Guppy and Shepard 2001; Opler and Wright 1999; Pyle 2002).
This species has several named subspecies including I.i. pembina and montis, which occur in Washington. The case for subspecific designation of blackmorei seems to be based primarily on distribution, life history traits, and reduced black spotting on the underside of the wings. The subspecies can only be separated in series as there is some overlap. Many specimens of blackmorei have little or no dark spotting in the underside of the hind wing and forwing spotting is much reduced when compared to pembina and montis. The ground color on the underside of the wings is also generally much lighter in blackmorei than in either pembina or montis. This, along with blackmorei’s geographic isolation is the basis for it’s subspecific designation.
Icaricia icarioides blackmorei, as currently designated, occurs in both lowland and subalpine habitats. There are no records of I.i. blackmorei within approximately 50 miles of its nearest likely sister subspecies montis or fenderi. Any Icaricia icarioides found in the Puget Trough, Olympic Mountains, or Vancouver Island lowlands is blackmorei.
Type locality
The holotype of I.i. blackmorei is from Goldstream, Vancouver Island, British Columbia, and the specimen is in the US National Museum of Natural History (Guppy personal communication).
Material studied
All sorted specimens in the Oregon State Arthropod Collection were examined. The Oregon State Arthropod Collection is the most complete collection of Northwestern U.S. Lepidoptera and it contains I.i.blackmorei from several areas. It also contains many Canadian specimens. Additional specimens reside at the American Museum of Natural History, Canadian National Collection, Natural History Museum of Los Angeles County, Royal British Columbia Museum, and the Burke Museum of Natural History and Culture. Information from these specimens is included in descriptions of the butterfly’s distribution. Specimens are also known to be at the M.T. James Museum at Washington State University.
Etymology
Nabokov named the genus in a play of words on the type species icarioides and a European genus, Aricia, which he believed to be affiliated (Guppy and Shepard 2001). Biosduval named the species, which he originally placed in Lycaena (Boisduval 1852) after the similar European L. icarus (Guppy and Shepard 2001). The subspecific designation blackmorei by Barnes and McDunnough was in honor of the collector of the specimens, Lepidopterist E.H. Blackmore (Guppy and Shepard 2001). Several common names are in use including Blackmore’s blue (Miller 1992) and Puget blue (Pyle 1989). The species is also referred to as the common blue (Miller 1992), lupine blue, Icarioides blue, and Boisduval’s blue (Pyle 2002).
Ecology
The primary host plant in lowland Puget Trough grasslands is Lupinus albicaulis, though it may use L. lepidus as well (Pyle 2002). It is also known to use L. latifolius in subalpine meadows in the Olympic mountains (Pyle 1989), although the alpine populations of blackmorei may be a different unnamed subspecies. Other populations of I. icarioides tend to strongly prefer one of the available lupines for oviposition even if multiple species are available (Downey and Dunn 1964). While most butterfly species are quite plastic in their nectar requirements, I.i. blackmorei has an unusual dependence on its larval host plant for adult nectar sources as well. This means that their distribution is entirely tied to the host lupine. The life history of this butterfly is often assumed to be similar to the extensively studied and closely related I.i. fenderi in Oregon’s Willamette Valley.
"Nectaring" behavior (landing and extending the proboscis in a probing manner) by I.i. blackmorei is highly unusual. In an unpublished study of nectaring behavior Hays et al. reported that most nectaring was performed on unopened flower heads of L. albicaulis. It is unclear what the butterflies were feeding on in these cases. The unexplained nectaring behavior is bizarre and it is unclear how the butterfly meets its energy requirements as an adult. This has not been observed in other subspecies of I. icarioides, including the closely related I.i. fenderi which needs nectar from other sources (Schultz and Dlugosch 1999; Schultz et al. 2003).
Both I.i. blackmorei and I.i. fenderi are known to spend most of their time on or near lupine plants, but Fender’s blue is not known to depend on lupines as a source for nectaring. Schultz (1998), in a study of Fender's blues in the Willamette Valley, found that adults were rarely seen at distances greater than 200m from host lupines and spent between 93% and 98% of their time less than 10m from lupine. Two of 74 individuals moved between patches separated by 150m, so some movement does take place beyond 100m. This evidence from I.i. fenderi and several other specialist lycaenids (New 1993) indicates I.i. blackmorei is probably quite sedentary with high intrinsic barriers to dispersal, and thus vulnerable to piecemeal extinction when metapopulations are fragmented. The exceptionally close relationship between I.i. blackmorei and its host makes it likely that it is at least as sedentary as the Fender’s blue.
Ants play a role in the development of larvae but it is unclear to what extent this is important for larval development. In other subspecies ants are important in protecting larvae against predators and parasitoids. Eleven species of ant were observed participating in this mutualistic interaction with I. icarioides in one study (Downey 1962). Some have observed that ants are relatively unimportant in the life history of I.i. blackmorei because of the low numbers of active ants early in the year when the larvae are active (Hammond, personal communication). Many other lycaenids are obligate myrmecophiles of various ant species. They often trade a sweet exudate and pheremonal cues for protection from predators and parasites (Agrawal and Fordyce 2000; New 1993). The role of ants in the life history of I. i. blackmorei is uncertain, but it could be crucial for the persistence of the subspecies, as has proven to be the case for other myrmecophilic butterflies (Ravenscroft 1990).
In areas currently occupied by I.i. blackmorei there is a high density of L. albicaulis and the vegetation is usually at least partly native prairie dominated by Roemer’s fescue (Festuca roemeri, formerly F. idahoensis in part), though in some areas it is degraded by Scotch broom, vehicular use, and tall exotic grasses. I.i. blackmorei is less stringent in its vegetation requirements than several other rare grassland butterflies in the Willamette Valley-Puget Trough-Georgia Basin ecoregion. Researchers found that the butterfly did not require continuous high quality native prairie and could still use areas with 28-60% cover of non-native grasses and forbs (Hays, Potter, Thompson, and Dunn, unpublished data). This suggests that in areas that are somewhat degraded with non-native grasses but still retaining good populations of L. albicaulis the species should be able to survive.
Distribution and conservation
I.i.blackmorei’s current known distribution is somewhat limited. They are found in some subalpine meadows in the northeast Olympic mountains (this may be an as yet unnamed subspecies according to Pelham) and from several locations in the Puget Trough on native grasslands (Hinchliff 1996, specimen data, personal sight records) [Distributional details are omitted for conservation reasons]. All areas known to have significant patches of Lupinus albicaulis in the Puget Trough lowlands appear to still be occupied by the butterfly.
This butterfly has several healthy populations, some of which are in Olympic National Park or adjacent wilderness areas, thus giving at least subalpine populations protection from most anthropogenic habitat destruction. It has been assumed that the invasion of grasslands by Scot’s broom (Cytisus scoparius) has led to the extirpation of I.i.blackmorei from all lowland areas on Vancouver Island (Guppy and Shepard 2001). It is in some danger of losing all of its lowland populations in Washington as well.
Protection of the remaining Lupinus albicaulis areas as well as appropriate management of those sites to ensure lupine persists will be key to conservation of the species in the Puget Trough lowlands. It is important to control invasive plants that compete with or shade out the lupine. It may also be necessary to induce some disturbance, particularly fire and mowing with biomass removal, as lupine generally responds well to these disturbances. Fire must be used very cautiously or it can have a negative impact on the butterfly by causing direct mortality to both adults and larvae. Burning 1/3 or less of the habitat every year, but more than 1/6 every other year, was recommended as the strategy that best maximized the population growth rate of I.i fenderi in population models (Schultz and Crone 1998). A similar strategy may work well for I.i. blackmorei.
The direct and indirect effects of management actions on grassland vegetation must be considered. Lupinus albicaulis seems to respond positively to late summer fire, mowing, graminoid specific herbicide application, and other disturbances (unpublished observations). It is also likely to respond positively to available nitrogen reduction efforts (Maron and Jefferies 2001; Wilson and Gerry 1995). The importance of various non-Lupinus nectar sources, the impacts of spring or late fall fire prescribed fire, the response of non-Lupinus nectar sources to fire, as well as the impacts of these treatments on diapause success are unknown. This argues for a conservative adaptive management approach to restoration and invasive species management.
Because the population structure of extant populations has likely changed, and the number of populations has likely dramatically declined due to habitat loss, it is important to retain all extant populations. To ensure this, all populations must have unburned, healthy populations of Lupinus albicaulis available. Due to the current separation of Lupinus albicaulis populations recolonization of extirpated populations is unlikely without human help (Char and Boersma 1995).
The minimum patch size necessary to maintain a population in isolation is uncertain. Schultz and Crone (1999) indicate that an area larger than 2ha is necessary to support I.i. fenderi. Several I.i. blackmorei populations are found in dry “kettles” or other minor topographic relief left behind by the retreating mass of the Vashon glacier. The role of this topographic variation in creating different microclimates and soil moisture regimes may be important for the long term persistence of I.i. blackmorei in a variable climate. Evidence from other species indicates this can be important for long term persistence of butterflies with sedentary populations (Dobkin et al. 1987; McLaughlin et al. 2002; Weiss et al. 1988). The original metapopulation structure of I.i. blackmorei has likely been disrupted by the fragmentation of the prairies (Char and Boersma 1995). The role this should play in the management of the species for conservation is unclear but it will probably involve physical translocations in areas where it is not possible to maintain habitat corridors.
References
Agrawal, A. A., and J. A. Fordyce. 2000. Induced direct defence in a lycaenid-ant association: the regulation of a resource in a mutualism. Proceedings of the Royal Society of London Series B- Biological Sciences 267:1857-1861.
Bálint, Z., and K. Johnson. 1997. Reformation of the Polyommatus section with a taxonomic and biogeographic overview (Lepidoptera, Lycaenidae, Polyommatini). Neue Entomologische Nachrichten 40:3-68.
Black, S. H., M. Shepard, and M. M. Allen. 2001. Endangered invertebrates: the case for greater attention to invertebrate conservation. Endangered Species UPDATE 18 (2):42-50.
Boisduval, J. A. 1852. Lepidopteres de la California. Annales de la Sociaetae Entomologique de France 10 (2):275-324.
Char, P., and P. D. Boersma. 1995. The effects of prairie fragmentation on butterfly species in western Washington. Seattle: University of Washington.
Crawford, R. C., and H. Hall. 1997. Changes in the south Puget prairie landscape. In Ecology and conservation of the south Puget Sound prairie landscape, edited by P.V. Dunn and K. Ewing, 11-16. Seattle, WA: The Nature Conservancy.
Dobkin, D. S., I. Olivieri, and P. R. Ehrlich. 1987. Rainfall and the interaction of microclimate with larval resources in the population dynamics of checkerspot butterflies (Euphydryas editha) inhabiting serpentine grassland. Oecologia 71:161-166.
Downey, J. C. 1962. Myrmecophily in Plebejus (Icaricia) icarioides (Lepid.: Lycaenidae). Entomological News 73 (3):57-66.
Downey, J. C., and D. B. Dunn. 1964. Variation in the Lycaenid butterfly Plebejus icarioides. III. Additional data on food plant specificity. Ecology 45 (1):172-178.
Emmel, T. C., ed. 1998. Systematics of western North American butterflies. Gainsville, FL: Mariposa Press.
Gorbunov, P. Y. 2001. The Butterflies of Russia: Classification, Genitalia, Keys for Identification (Lepidoptera: Hesperioidea and Papilionoidea), Institute of Plant and Animal Ecology, Russian Academy of Sciences, Ekaterinburg, Russia.
Guppy, C. S., and J. H. Shepard. 2001. Butterflies of British Columbia: including western Alberta, southern Yukon, the Alaska panhandle, Washington, northern Oregon, northern Idaho, and northwestern Montana. Vancouver, BC: University of British Columbia Press.
Hinchliff, J. 1996. The distribution of the butterflies of Washington. Corvallis, OR: Oregon State University Bookstore.
Hodges, R. W., T. Dominick, D. R. Davis, D. C. Ferguson, J. G. Franclemont, E. G. Munroe, and J. A. Powell, eds. 1983. Check list of the Lepidoptera of America north of Mexico including Greenland. London: E.W. Classey Limited.
Launer, A. E., and D. D. Murphy. 1994. Umbrella species and the conservation of habitat fragments: a case of a threatened butterfly and a vanishing grassland ecosystem. Biological Conservation 69:145-153.
Leonard, W. P., and L. Hallock. 1997. Herpetofauna of south Puget Sound prairie landscape. In Ecology and conservation of the south Puget Sound prairie landscape, edited by P.V. Dunn and K. Ewing, 65-74. Seattle, WA: The Nature Conservancy.
Maron, J. L., and R. L. Jefferies. 2001. Restoration of enriched coastal grasslands: effects of mowing on species richness, productivity and nitrogen retention. Ecological Applications 11:1088-1100.
McLaughlin, J. F., J. J. Hellmann, C. L. Boggs, and P. R. Ehrlich. 2002. The route to extinction: population dynamics of a threatened butterfly. Oecologia 132:538-548.
Miller, J. Y. 1992. The common names of North American butterflies. Washington, DC: Smithsonian Institution Press.
New, T. R. 1993. Conservation biology of the Lycaenidae (Butterflies). Vol. 8, Occasional Paper of the IUCN Species Survival Commision. Gland, Switzerland: IUCN.
———. 1997. Are Lepidoptera an effective 'umbrella group' for biodiversity conservation. Journal of Insect Conservation 1:5-12.
Opler, P. A., and A. D. Warren. 2003. Butterflies of North America. 2. scientific names list for butterfly species of North America, north of Mexico.
Opler, P. A., and A. B. Wright. 1999. Western butterflies, Peterson Field Guides. New York: Houghton Mifflin.
Pollard, E., and T. J. Yates. 1993. Monitoring butterflies for ecology and conservation: the British butterfly monitoring scheme. Edited by F.B. Goldsmith and E. Duffy, Conservation Biology Series. London: Chapman and Hall.
Pyle, R. M. 1989. Washington Butterfly Conservation Status Report and Plan. Gray's River, WA: Washington Department of Fish and Wildlife.
———. 2002. The butterflies of Cascadia. Seattle, WA: Seattle Audubon Society.
Ravenscroft, N. O. M. 1990. The ecology and conservation of the silver-studded blue Plebejus. Biological Conservation 53:21-36.
Remsberg, M. 2000. Summary report of LCTA butterfly surveys conducted on Fort Lewis, WA. Fort Lewis, WA: United States Army.
Rogers, R., D. Norman, and D. Rolph. 1997. The status of neotropical migrant birds in the prairie landscape. In Ecology and Conservation of the south Puget sound prairie landscape, edited by P.V. Dunn and K. Ewing. Seattle, WA: The Nature Conservancy.
Rogers, R. E. 2000. The status and micro-habitat selection of streaked horned lark, western bluebird, Oregon vesper sparrow, and western meadowlark in western Washington. Masters Thesis, Master of Environmental Studies Program, The Evergreen State College, Olympia.
Ryan, L. A. 1997. Ecology of the western gray squirrel in the south Puget Sound. In Ecology and Conservation of the south Puget Sound prairie landscape, edited by P.V. Dunn and K. Ewing, 35-42. Seattle, WA: The Nature Conservancy.
Samways, M. J. 1994. Insect conservation biology. Edited by F.B. Goldsmith and E. Duffy, Conservation Biology Series. London: Chapman and Hall.
Schultz, C. B., and E. E. Crone. 1998. Burning prairie to restore butterfly habitat: a modeling approach to management tradeoffs for the Fender's blue. Restoration Ecology 6 (3):244-252.
Schultz, C. B., and K. M. Dlugosch. 1999. Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia 119:231-238.
Schultz, C. B., P. C. Hammond, and M. V. Wilson. 2003. Biology of the Fender's Blue Butterfly (Icaricia icarioides fenderi Macy), an Endangered Species of Western Oregon Native Prairies. Natural Areas Journal 23 (1):61-71.
Vaughan, M., and S. H. Black. 2002a. Petition to List the Mardon Skipper Butterfly (Polites mardon) as an endangered species under the U.S. Endangered Species Act: The Xerces Society, Gifford Pinchot Task Force, The Northwest Environmental Defense Center, Center for Biological Diversity, Oregon Natural Resources Council, Friends of the San Juan's, Northwest Ecosystem Alliance.
———. 2002b. Petition to List the Taylor's (Whulge) checkerspot (Euphydryas editha taylori) as an endangered species under the U.S. Endangered Species Act. Portland, OR: The Xerces Society, Gifford Pinchot Task Force, The Northwest Environmental Defense Center, Center for Biological Diversity, Oregon Natural Resources Council, Friends of the San Juan's, Northwest Ecosystem Alliance.
Weiss, S. B., D. D. Murphy, and R. R. White. 1988. Sun, slope, and butterflies: topographic determinants of habitat quality for Euphydryas editha. Ecology 69 (5):1486-1496.
Wilson, S. D., and A. K. Gerry. 1995. Strategies for mixed-grass prairie restoration: herbicide, tilling, and nitrogen manipulation. Restoration Ecology 3 (4):290-298.
Page author: Daniel N. Grosboll. dangrosboll@earthlink.net
(This document was completed as part of an Individual Learning Contract for the MES Program at The Evergreen State College, with faculty advisor John T. Longino.)
Last modified: 31 August 2005.